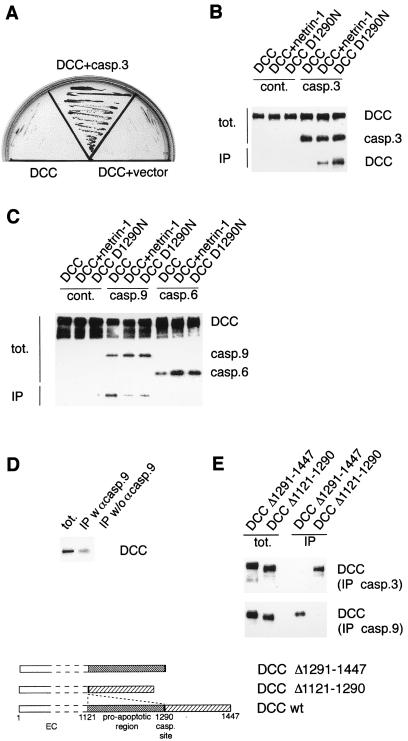
Figure 3.
Interaction of caspase-3 and caspase-9 with the intracytoplasmic domain of DCC. (A) Two-hybrid screening of DCC-IC revealed interaction with caspase-3. Y190 yeast transformed with a GAL4 DNA binding domain-DCC-IC fusion (DCC) were transformed with either a mock plasmid containing the GAL4 transcriptional activation domain AD (DCC + vector) or a similar construct fused to a brain cDNA library. Cells were then allowed to grow in the absence of leucine, tryptophan, and histidine and in the presence of 55 mM 3-amino-1,2,4-triazole. The clone corresponding to caspase-3 is shown (DCC + caspase-3). (B) Coimmunoprecipitations were performed on 293T cells cotransfected with pcDNA-DNcasp3 (casp.3) or pcDNA-3 (cont.) and pDCC-CMV-S, pDCC-CMV.D1290N, or pDCC-CMV-S + pGNET1myc, the netrin-1-encoding plasmid. To obtain a similar expression of the protein DCC in the different sample, half of the plasmid pDCC-CMV-S was used when cotransfected with the netrin-1-expressing vector because the presence of DCC ligand leads to increased stability of DCC (13). Anti-caspase-3 antibody was used to immunoprecipitate caspase-3, and binding was revealed by Western blot by using anti-DCC antibody. (C) Same as in B except that pcDNA-DNcasp9 and pcDNA-DNcasp6 were used. In this case, anti-Flag antibody was used to immunoprecipitate DNcaspase-9 and DNcaspase-6. (D) Immunoprecipitation of DCC by caspase-9 was performed by using IMR-32 cells. The pull-down was performed with or without anti-caspase-9 antibody. (E) Coimmunoprecipitations were performed, respectively, as in B and C on 293T cotransfected with pcDNA-DNcasp3 or pcDNA-DNcasp9 in the presence of either pDCC-CMV-Δ1121–1290 or pDCC-CMV-Δ1290–1447. tot., immunoblots performed on the lysate before addition of the antibodies allowing the pull out; IP, immunoblots by using an anti-DCC antibody on samples derived from the pull-downs. In B–E, dominant negative mutants of caspases were chosen instead of tagged wild-type because overexpression of wild-type caspase drove massive 293T cell death.