Abstract
Azithromycin (AZM) has shown promising anti-inflammatory properties in chronic obstructive pulmonary diseases, and clinical studies have presented an improvement in the respiratory condition of cystic fibrosis (CF) patients. The aim of this study was to investigate, in human airway cells, the mechanism by which AZM has beneficial effects in CF. We demonstrated that AZM did not have any anti-inflammatory effect on CF airway cells but restored Cl− efflux.
Cystic fibrosis (CF) is the most common genetic disorder in Caucasian populations. CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a chloride (Cl−) channel (5). This pathology affects many organs, but lung disease is the foremost cause of morbidity and mortality. In CF airway epithelium, sustained inflammation is due mainly to an increase in the transcription nuclear factor κB (NF-κB) activity, which leads to an increase in interleukin 8 (IL-8) production (6, 7). Since macrolides can reduce inflammatory markers in some pulmonary diseases, azithromycin (AZM) has been used to treat CF patients, but the molecular mechanisms remain unknown (1). This study investigates the effects of AZM on inflammatory pathways and on Cl− efflux that could explain benefits observed in CF patients.
First, we have demonstrated that the physiological concentration of AZM (10 μg/ml; 16 h) is not able to reduce IL-8 secretion and NF-κB activity in CF bronchial epithelial cells (IB3-1 and CFBE41o−) and in primary human bronchial glandular (HBG) cells treated with a specific inhibitor of CFTR activity (CFTRInh-172, 10 μM) (4). These results for the NF-κB pathway were obtained by different approaches, using a multiplex approach, Western blotting, and luciferase activity. In contrast, in non-CF cells (S9, 16HBE14o−, and HBG cells), AZM significantly reduced IL-8 secretion and NF-κB activity (data not shown).
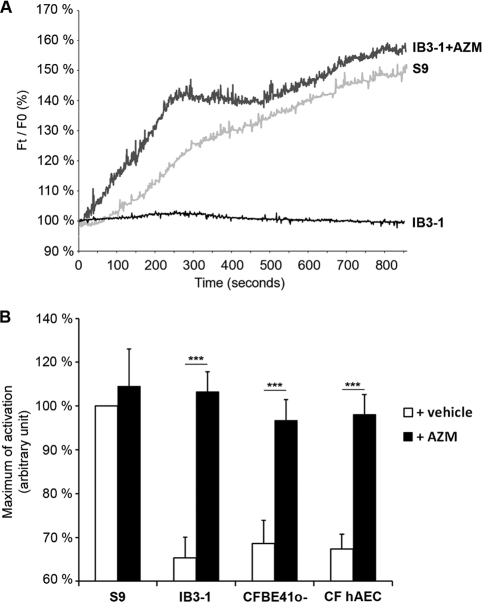
In the second part of this work, we have consolidated and also extended results obtained from Oliynyk (3). In our study, contrary to the case with erythromycin, AZM was able to significantly activate Cl− efflux in two CF human bronchial epithelial cell lines and in CF human primary airway epithelial cells (CF hAEC, from Epithelix) (Fig. 1). These results were obtained and quantified by two different methods [6-methoxy-N-(3-sulfopronyl)-quinolinium (SPQ) probe and Premo Halide Sensor method]. Moreover, the addition of CFTRInh-172 completely abolished Cl− restoration, suggesting that AZM is acting only through CFTR channel activity (data not shown). The rapid increase of Cl− activity (less than 5 min) observed in all used cell models suggests that AZM could act mainly as a potentiator of the F508del CFTR protein already present at the apical membrane of cells.
FIG. 1.
Effect of AZM (10 μg/ml) on chloride efflux in CF airway epithelial cells (IB3-1, CFBE41o−, and CF hAEC) and in non-CF airway cells (S9 cells). (A) Cells were loaded with SPQ probe, and fluorescence was measured every second after treatment with AZM. Data are relative fluorescence Ft/F0, where “Ft” is the fluorescence intensity at time T and “F0” is the fluorescence at time zero. (B) The histogram represents the means of activation at 600 s of 35 cells in 5 independent experiments. ***, P < 0.001.
In the literature, numerous data have shown that the most important defect in CF is in a lack of Cl− conductance. Therefore, restoring this Cl− activity may have a major beneficial clinical effect in the CF patient. The restoration of Cl− efflux could partially explain why AZM is involved in the correction of airway surface liquid composition, the increase of transepithelial electrical resistance affecting the processing of airway epithelial tight junction proteins and consequently the reduction in the binding of bacteria (2, 8). Thus, the involvement of AZM-associated Cl− efflux observed in these studies remains to be determined.
In conclusion, the present findings support the hypothesis that AZM is implicated in the activation of the Cl− efflux in CF airway epithelial cells and thus may have a beneficial effect in CF patients.
Acknowledgments
Inserm, Vaincre La Mucoviscidose, and UPMC Univ. Paris 06 supported this work.
We thank Cormac Jennings for his critical reading of the manuscript and the IFR65 multiplex Core Facility. hAEC were provided by Epithelix Sarl (Geneva, Switzerland), and we thank Dieter C. Gruenert for providing CFBE41o− cells.
Footnotes
Published ahead of print on 10 January 2011.
REFERENCES
- 1.Clement, A., et al. 2006. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax 61:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee, A., et al. 1999. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol. 277:L204-1217. [DOI] [PubMed] [Google Scholar]
- 3.Oliynyk, I., et al. 2009. Azithromycin increases chloride efflux from cystic fibrosis airway epithelial cells. Exp. Lung Res. 35:210-221. [DOI] [PubMed] [Google Scholar]
- 4.Perez, A., et al. 2007. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L383-L395. [DOI] [PubMed] [Google Scholar]
- 5.Riordan, J. R., et al. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. (Erratum, 245:1437.) [DOI] [PubMed] [Google Scholar]
- 6.Tabary, O., et al. 2006. Calcium-dependent regulation of NF-(kappa)B activation in cystic fibrosis airway epithelial cells. Cell Signal. 18:652-660. [DOI] [PubMed] [Google Scholar]
- 7.Vij, N., S. Mazur, and P. L. Zeitlin. 2009. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS One 4:e4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousef, A. A., and A. Jaffe. 2010. The role of azithromycin in patients with cystic fibrosis. Paediatr. Respir. Rev. 11:108-114. [DOI] [PubMed] [Google Scholar]