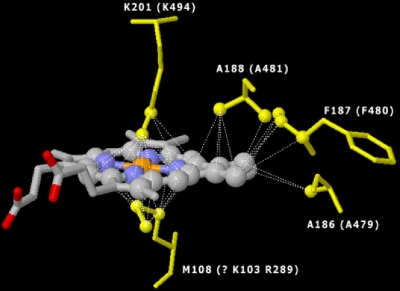
FIG. 6.
Model of the FoxC putative heme B binding domain (orange, Fe; blue, N), indicating the spatial location of heme B relative to axial coordinating residues. The model shows lysine (K201) and methionine (M108) axial coordination for the EBDH crystal and corresponding lysine (K494) and arginine (R289) or lysine (K103) for the FoxC model. The model also shows conserved alanine (A188 and A186 for the EDBH crystal; A481 and A479 for FoxC) and phenylalanine (F187 for the EDBH crystal; F480 for FoxC).