Abstract
Pneumococcal disease continues to cause substantial morbidity and mortality among the elderly. Older adults may have high levels of anticapsular antibody after vaccination, but their antibodies show decreased functional activity. In addition, the protective effect of the pneumococcal polysaccharide vaccine (PPV) seems to cease as early as 3 to 5 years postvaccination. Recently, it was suggested that PPV elicits human antibodies that use predominantly VH3 gene segments and induce a repertoire shift with increased VH3 expression in peripheral B cells. Here we compared VH3-idiotypic antibody responses in middle-aged and elderly subjects receiving PPV as initial immunization or revaccination. We studied pre- and postvaccination sera from 36 (18 vaccine-naïve and 18 previously immunized subjects) middle-aged and 40 (22 vaccine-naïve and 18 previously immunized subjects) elderly adults who received 23-valent PPV. Concentrations of IgGs to four individual serotypes (6B, 14, 19F, and 23F) and of VH3-idiotypic antibodies (detected by the monoclonal antibody D12) to the whole pneumococcal vaccine were determined by enzyme-linked immunosorbent assay (ELISA). PPV elicited significant IgG and VH3-idiotypic antibody responses in middle-aged and elderly subjects, regardless of whether they were vaccine naïve or undergoing revaccination. Age did not influence the magnitude of the antibody responses, as evidenced by similar postvaccination IgG and VH3 antibody levels in both groups, even after stratifying by prior vaccine status. Furthermore, we found similar proportions (around 50%) of elderly and middle-aged subjects experiencing 2-fold increases in VH3 antibody titers after vaccination. Age or repeated immunization does not appear to affect the VH3-idiotypic immunogenicity of PPV among middle-aged and elderly adults.
Streptococcus pneumoniae is the leading cause of bacterial pneumonia and bacterial meningitis in the United States, resulting in 175,000 hospitalizations and 7,000 to 12,000 deaths annually. Groups with the highest incidences of pneumococcal infection include the very young, the elderly, persons who are immunocompromised, smokers, and certain other demographic groups (2, 8). In persons 65 years or older, the incidence of invasive pneumococcal disease (IPD) is around 50 per 100,000 individuals per year and is associated with a case fatality rate of 20%, whereas among those aged 85 years or older, the fatality rate increases to 40% (2, 34).
The Advisory Committee on Immunization Practices recommends vaccinating all adults aged 65 years or older with the 23-valent pneumococcal polysaccharide vaccine (PPV). One-time revaccination for this age group is also recommended if subjects received their first dose ≥5 years previously and before the age of 65 years (6). A recent meta-analysis provided evidence supporting the recommendation for PPV to prevent IPD in adults. However, it did not provide compelling evidence to support the routine use of PPV to prevent all-cause pneumonia or mortality (15). In addition, significant protection against IPD seems to be lost as early as 3 to 5 years after vaccination in persons older than 65 years (28, 29).
A common surrogate for antibody-mediated protection is the measurement of postvaccination IgG antibody to capsular polysaccharides contained in PPV. Validation of this measure may be disputed given the fact that even adequate IgG concentrations in the elderly may have significant reductions in antibody functional activity toward pneumococcal polysaccharide antigens (25). Molecular characterization of the immune response to pneumococcal polysaccharides is seldom performed in clinical vaccine studies (24); however, there is a large body of literature on this subject (3, 5, 7, 22, 38). Recent studies have demonstrated that PPV stimulates increased expression of variable region heavy chain family 3 (VH3) genes in peripheral B cells from immunocompetent subjects, yielding serum polysaccharide-specific antibodies and/or B cells that express VH3 (1, 7, 32, 33). VH3 responses may also correlate with functional activity of antipneumococcal antibodies (3).
Previous studies on gene expression of the total circulating B-cell population demonstrated a shift toward VH4 and VH1 expression in aging humans, compared with predominant VH3 expression in young subjects (35). This repertoire shift has been postulated as a possible mechanism of decreased pneumococcal anticapsular antibody function in older populations. In this regard, a preliminary report (30) found lower levels of VH3-idiotypic antibody responses to capsular polysaccharides from serotype 4, but not serotype 14, in the elderly than in young individuals. A subsequent study (11) of the VH gene repertoire of human peripheral B cells specific for these two capsular polysaccharides (4 and 14) revealed that the responses in both age groups were dominated by the VH3 gene family (>90%). The VH1, VH4, and VH5 gene families were also isolated from both groups, but they constituted <10% of the total heavy chain repertoire. Given the attractiveness of the study of VH3-idiotypic antibody responses to assess the immunogenicity of pneumococcal polysaccharide antigens and the need for further studies on its role in aging, we evaluated IgG and VH3-idiotypic antibody responses after administration of PPV in sera from a subset of vaccine-naïve and revaccinated middle-aged and elderly subjects enrolled in a pneumococcal vaccine clinical trial (19).
MATERIALS AND METHODS
Studies were done with pre- and postvaccination sera from 36 (18 vaccine-naïve and 18 previously immunized subjects) middle-aged (50 to 64 years) and 40 (22 vaccine-naïve and 18 previously immunized subjects) elderly (≥65 years) adults who received one intramuscular dose of the 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23; Merck, West Point, PA). This vaccine contains 25 μg of each of the following polysaccharide types per 0.5 ml: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33.
Study subjects were participants in a multisite study evaluating the safety and immunogenicity of primary vaccination and revaccination with PPV and were recruited and vaccinated at the Houston Veterans Affairs Medical Center under a protocol that was approved by the Institutional Review Board, Baylor College of Medicine, as previously described (19). Subjects were included based on the availability of sera. Reimmunized subjects had received the initial vaccine 3 to 5 years previously as part of their routine medical care. Blood samples were obtained before and 4 weeks after initial vaccination or revaccination. Sera were separated from whole-blood samples by centrifugation and were stored at −70°C until analysis. For the current study, all samples were reassayed as described below.
Isotype expression of antibodies.
The pre- and postvaccination concentrations of IgGs to pneumococcal capsular polysaccharides of types 6B, 14, 19F, and 23F were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) as described elsewhere (36). We decided to study four representative serotypes that are reported to cause a significant number of cases of IPD among middle-aged and elderly persons in the United States (37).
Serum samples were preabsorbed with 5 μg/ml pneumococcal cell wall polysaccharide (CWPS; Statens Serum Institut) and 5 μg/ml type 22F CPS (ATCC) for 30 min at room temperature, following the World Health Organization guidance protocol for pneumococcal antibody ELISA (36) (this methodology differs from the one described for the main study, which used serotypes 25 and 72 for adsorption [19]). Dilutions of a laboratory reference serum (pool 89-SF; Food and Drug Administration) were included in every ELISA plate. All serum samples from each individual were studied concurrently.
Idiotype expression of antibodies.
The expression of VH3 antibodies to the pneumococcal vaccine in pre- and postvaccination sera was determined by ELISA as described elsewhere (1). In brief, 96-well polystyrene plates (Costar; Corning Glass Works) were coated with 10 μg/ml of whole PPV in phosphate-buffered saline (PBS) for 5 h at 37°C and then kept overnight at 4°C. Before use, plates were blocked with PBS containing 1% bovine serum albumin (Fisher Scientific) for 1 h and then washed with PBS containing 0.05% Tween 20 (Fisher Scientific). ELISA plates were incubated for 2 h at 37°C with CWPS-absorbed pre- and postvaccination serum samples diluted 1:3, beginning with an initial dilution of 1:10 in PBS. After incubation, the plates were washed and incubated for 1 h at 37°C with the murine monoclonal antibody D12, which binds to a variable region determinant expressed by certain antibodies encoded by VH3 genes, at a concentration of 5 μg/ml. The binding of the D12-bound serum antibodies was detected with alkaline phosphatase-labeled goat anti-mouse IgG1 (Southern Biotech). The plates were then washed and developed with p-nitrophenyl phosphate, and absorbances and titers were calculated.
Statistics.
Geometric mean concentrations (GMCs) of IgGs to individual capsular polysaccharides, geometric mean VH3 titers to the whole vaccine, and corresponding 95% confidence intervals (95% CI) were calculated. All antibody concentrations and titers were log10 converted before any statistical comparisons were made. The pre- and postvaccination levels were compared between groups (middle-aged and elderly) by unpaired Student's t test. The pre- and postvaccination levels within each group were compared by paired Student's t test. Stratification by vaccination status was also studied, and comparison of multiple subgroups was done with analysis of variance. Pearson's correlation coefficient was calculated to measure the level of association between IgG and VH3-idiotypic antibody responses (defined as postvaccination log-converted antibody levels − prevaccination log-converted antibody levels). Multiple comparisons were accounted for by using Bonferroni correction. Statistical analyses were implemented using STATA 9.2 (StataCorp., College Station, TX), and P values of <0.05 were considered significant.
RESULTS
IgG antibody response.
GMCs of IgGs to individual pneumococcal capsular polysaccharides (serotypes) are shown in Table 1 according to age group and vaccination status. Pre- and postvaccination IgG levels were similar between the middle-aged and elderly groups. When stratified by vaccine status, prevaccination IgG levels were higher in previously immunized subjects than in vaccine-naïve subjects in both groups and for all serotypes; however, this difference was statistically significant only for serotype 14 for both the middle-aged and elderly groups (P < 0.03 for both comparisons). Despite these differences in prevaccination levels, postvaccination IgG levels were similar in all subgroups. The middle-aged and elderly groups showed significant increases (within-group comparisons) in IgG levels for all four serotypes (P < 0.01 for all comparisons). Stratified analysis also showed significant IgG increases in all subgroups for all serotypes (P < 0.05 for all comparisons).
TABLE 1.
GMCs of IgG antibodies to individual pneumococcal capsular polysaccharides in pre- and postvaccination sera from middle-aged and elderly subjects according to vaccine status
| Group (n) | IgG GMC (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Serotype 6B |
Serotype 14 |
Serotype 19F |
Serotype 23F |
|||||
| Prevaccination | Postvaccinationb | Prevaccination | Postvaccinationb | Prevaccination | Postvaccinationb | Prevaccination | Postvaccinationb | |
| Middle-aged subjects (36) | 1.97 (1.46-2.65) | 3.03 (2.35-3.92) | 3.26 (2.38-4.47) | 9.33 (6.33-13.74) | 2.09 (1.66-2.64) | 3.46 (2.55-4.70) | 1.21 (0.92-1.59) | 2.23 (1.65-3.00) |
| Vaccine-naïve subjects (18) | 1.68 (1.17-2.40) | 2.73 (2.01-3.71) | 2.06 (1.59-2.67) | 11.20 (5.51-22.06) | 1.85 (1.31-2.61) | 3.14 (1.82-5.41) | 0.94 (0.63-1.39) | 2.04 (1.36-3.05) |
| Previously vaccinated subjects (18) | 2.30 (1.39-3.80) | 3.36 (2.17-5.22) | 5.17a (3.10-8.63) | 7.89 (5.20-11.97) | 2.36 (1.68-3.31) | 3.81 (2.70-5.36) | 1.57 (1.09-2.26) | 2.43 (1.51-3.93) |
| Elderly subjects (40) | 2.21 (1.67-2.93) | 3.18 (2.26-4.49) | 3.89 (2.81-5.41) | 7.45 (5.19-10.68) | 2.59 (2.07-3.24) | 4.02 (3.03-5.33) | 1.24 (0.97-1.59) | 2.73 (1.94-3.85) |
| Vaccine-naïve subjects (22) | 1.99 (1.34-2.97) | 3.32 (1.91-5.77) | 2.67 (1.78-4.00) | 6.65 (3.97-11.14) | 2.25 (1.67-3.04) | 3.33 (2.17-5.09) | 1.07 (0.77-1.48) | 2.98 (1.75-5.08) |
| Previously vaccinated subjects (18) | 2.51 (1.63-3.85) | 3.02 (1.98-4.61) | 6.20a (3.80-10.11) | 8.55 (4.95-14.75) | 3.07 (2.15-4.40) | 5.06 (3.49-7.33) | 1.50 (1.02-2.19) | 2.46 (1.56-3.87) |
Significant difference (P < 0.03) in log-converted prevaccination concentrations (μg/ml) between previously vaccinated and vaccine-naïve subjects.
Significant increases (P < 0.05) in IgG concentrations (within-group comparisons) were seen for all groups.
VH3-idiotypic antibody response.
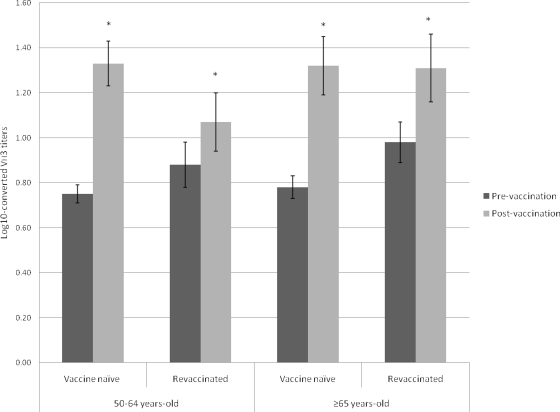
Pre- and postvaccination VH3 levels were similar between the middle-aged and elderly groups, even after stratification by vaccine status. Both groups showed significant increases (within-group comparisons) in VH3 antibody titers after vaccination with PPV (P < 0.01 for both comparisons) (Table 2). Stratified analysis also showed significant increases in VH3 levels in all subgroups (P < 0.03 for all comparisons) (Fig. 1). Comparable proportions of middle-aged and elderly patients experienced 2-fold increases in VH3 antibody titers (54.8% and 45.7%, respectively; P = 0.46). Although the differences were not statistically significant, larger proportions of vaccine-naïve subjects than revaccinated subjects had 2-fold increases in VH3 antibody titers (75% and 55% versus 33.3% and 33.3% for middle-aged and elderly patients, respectively) (P = 0.06).
TABLE 2.
Geometric mean VH3 antibody titers to pneumococcal polysaccharide vaccine in pre- and postvaccination sera from middle-aged and elderly subjects according to vaccine statusa
| Group (n) | Geometric mean VH3 antibody titer (95% CI) |
|
|---|---|---|
| Prevaccination | Postvaccinationb | |
| Middle-aged subjects (31) | 6.48 (5.10-8.24) | 16.12 (10.92-23.78) |
| Vaccine-naïve subjects (16) | 5. 65 (4.73-6.74) | 21.60 (13.45-34.70) |
| Previously vaccinated subjects (15) | 7.51 (4.66-12.13) | 11.80 (6.18-22.49) |
| Elderly subjects (35) | 7.38 (5.87-9.27) | 20.86 (13.27-32.78) |
| Vaccine-naïve subjects (20) | 6.06 (4.81-7.63) | 21.05 (11.26-39.35) |
| Previously vaccinated subjects (15) | 9.59 (6.19-14.84) | 20.60 (9.84-43.16) |
No significant differences in log-converted pre- or postvaccination titers were seen between middle-aged and elderly subjects.
Significant increases (P < 0.03) in log-converted VH3 antibody titers (within-group comparisons) were seen for all groups.
FIG. 1.
Log10-converted VH3-idiotypic antibody titers to pneumococcal polysaccharide vaccine in pre- and postvaccination sera from middle-aged and elderly subjects, stratified according to vaccination status. *, P < 0.05.
Correlations.
VH3-idiotypic antibody responses were correlated with IgG responses to serotypes 14 (r = 0.36; P = 0.03) and 23F (r = 0.45; P < 0.01). No significant correlations were observed for the other two serotypes.
DISCUSSION
In our study, age did not influence the magnitude or quality of the antibody responses elicited by the pneumococcal polysaccharide vaccine among either vaccine-naïve or previously immunized subjects. This was evidenced by similar postvaccination IgG and VH3 antibody levels in middle-aged and elderly groups even after stratifying by prior vaccine status. Furthermore, our study showed similar proportions of healthy elderly and middle-aged subjects experiencing 2-fold increases in VH3 antibody titers after receiving pneumococcal polysaccharide vaccine.
Several previous studies have shown IgG antibody responses to pneumococcal polysaccharides in elderly individuals similar to those of their younger counterparts (4, 17, 26, 27); however, these levels do not always accurately correlate with functional antibody activity (25) or with protection against pneumococcal disease (20). In addition, other studies have suggested that humoral responses among elderly subjects correlate better with their functional status than with their chronological age (4, 23). Our patients were all ambulatory subjects, without concomitant debilitating acute or chronic illness, who were expected to survive for 5 years after study enrollment (19). Therefore, our results on VH3 antibody responses among ambulatory elderly subjects may not be extrapolated to frail elderly patients.
As a vaccine composed of polysaccharide antigens, PPV induces a T-cell-independent response, and thus an anamnestic response (i.e., booster) is unlikely (31). Immunologic tolerance or hyporesponsiveness induced by prior polysaccharide antigen exposure constitutes a potential concern in considering revaccination with PPV (9, 21). Although prevaccination IgG levels in response to some serotypes were higher in previously immunized subjects, postvaccination levels actually reached similar levels in all subgroups. However, as a result of higher prevaccination titers, a smaller proportion of revaccinated subjects achieved a 2-fold-increased response. Our results are concurrent with those of the main study (19). In addition, in a different substudy, Manoff and collaborators showed that revaccination of adults aged ≥65 years was comparable to primary vaccination for inducing elevated and persistent functional (measured by opsonophagocytic assay), specific anticapsular antibodies; in this study, the results of the functional assays correlated with the IgG antibody responses (14). These results agree with those of earlier studies showing that revaccination elicits a significant response, but not one of greater intensity than that to the initial vaccination (10, 14, 16, 34) and, most importantly, indicate that any possible differences initially observed in the antibody responses of revaccinated and vaccine-naïve subjects do not persist over time. Among HIV-infected patients on highly active antiretroviral therapy (HAART), the VH3 antibody responses to polysaccharide antigens after revaccination were similar to those after initial vaccination (32). Similarly, our data indicate that adults aged 50 to 65 years or >65 years do mount significant serotype- and VH3-specific antibody responses after revaccination with PPV. However, we have not evaluated the functional activity of the VH3-specific antibodies, and correlation of VH3-specific antibodies with function, although supported by one small study (3), continues to be speculative.
We found a linear correlation between VH3 antibodies to the whole vaccine and IgG responses to two serotypes (14 and 23F). In a previous study (1) utilizing the same assay, antibodies to capsular polysaccharides of serotypes 3, 6B, and 14 did not yield VH3 determinants recognized by the D12 monoclonal antibody. The prior study evaluated antibody responses among a younger group of subjects. It is possible that there is a differential expression of VH3 epitopes among different serotypes that is influenced by age (11, 29).
We measured VH3 antibody responses with the use of the D12 monoclonal antibody and a whole-vaccine ELISA because we had previously demonstrated that the determination of whole-vaccine D12-positive antibodies is a reliable method of demonstrating pre-to-postvaccine increases in D12-positive antibodies (32). With this assay, 23 (35%) of 66 patients had no detectable VH3 antibody response. The D12 monoclonal antibody does not appear to recognize all VH3 family antibodies, and it is possible that by the use of a panel consisting of several monoclonal antibodies that recognize different VH3 epitopes, we would have been able to detect greater VH3 responses (1). Hence, we are limited in our ability to detect additional differences in age-related responses, responses based on prior immunization status, or correlation with IgG responses. In addition, we did not study any other VH gene families, which limits our ability to detect any subtle shifts in VH family use. A more complex PCR-based methodology would have increased our ability to better characterize the entire VH antibody repertoire (12, 39).
A decreased production of VH3 antibodies and a shift to the VH5 gene family after receiving PPV have been postulated as contributing factors to the increased susceptibility to S. pneumoniae infections among HIV-infected patients (1, 7, 32). Thus, it appears reasonable to hypothesize that the study of VH3 antibody responses may offer an additional approach to test the antibody response after administration of the pneumococcal polysaccharide vaccine. We demonstrated that vaccination with pneumococcal polysaccharide vaccine elicits significant IgG and VH3-idiotypic antibody responses in middle-aged and elderly subjects, whether they are vaccine naïve or undergoing revaccination 3 to 5 years after primary immunization. The VH3 antibody response was not impaired among elderly adults. Vaccination and revaccination with pneumococcal polysaccharide vaccine after 3 to 5 years appear to be effective strategies to elicit isotypic and VH3-idiotypic immunogenicity in middle-aged and elderly adults (10, 13, 16, 18, 27, 28, 34).
Acknowledgments
This work was supported by the Department of Veterans Affairs through Merit Review Funding (to D.M.M. and M.C.R.-B.).
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1.Abadi, J., et al. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of V(H)3 gene segment usage. J. Infect. Dis. 178:707-716. [DOI] [PubMed] [Google Scholar]
- 2.Artz, A. S., W. B. Ershler, and D. L. Longo. 2003. Pneumococcal vaccination and revaccination of older adults. Clin. Microbiol. Rev. 16:308-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxendale, H. E., and D. Goldblatt. 2006. Correlation of molecular characteristics, isotype, and in vitro functional activity of human antipneumococcal monoclonal antibodies. Infect. Immun. 74:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson, P. J., K. L. Nichol, J. O'Brien, P. Hilo, and E. N. Janoff. 2000. Immune function and vaccine responses in healthy advanced elderly patients. Arch. Intern. Med. 160:2017-2024. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., and L. A. Pirofski. 2004. New concepts in antibody-mediated immunity. Infect. Immun. 72:6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. 2002. Recommended adult immunization schedule—United States, 2002-2003. MMWR Morb. Mortal. Wkly. Rep. 51:904-908. [PubMed] [Google Scholar]
- 7.Chang, Q., J. Abadi, P. Alpert, and L. Pirofski. 2000. A pneumococcal capsular polysaccharide vaccine induces a repertoire shift with increased VH3 expression in peripheral B cells from human immunodeficiency virus (HIV)-uninfected but not HIV-infected persons. J. Infect. Dis. 181:1313-1321. [DOI] [PubMed] [Google Scholar]
- 8.Fedson, D. S., and D. M. Musher. 2004. Pneumococcal polysaccharide vaccine, p. 589-624. In S. A. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines. Elsevier, Philadelphia, PA.
- 9.González-Fernández, A., J. Faro, and C. Fernández. 2008. Immune responses to polysaccharides: lessons from humans and mice. Vaccine 26:292-300. [DOI] [PubMed] [Google Scholar]
- 10.Jackson, L. A., et al. 1999. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 281:243-248. [DOI] [PubMed] [Google Scholar]
- 11.Kolibab, K., S. L. Smithson, B. Rabquer, S. Khuder, and M. A. Westerink. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect. Immun. 73:7465-7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolibab, K., et al. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun. Ageing 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lackner, T. E., et al. 2003. Pneumococcal polysaccharide revaccination: immunoglobulin G seroconversion, persistence, and safety in frail, chronically ill older subjects. J. Am. Geriatr. Soc. 51:240-245. [DOI] [PubMed] [Google Scholar]
- 14.Manoff, S. B., et al. 2010. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. J. Infect. Dis. 201:525-533. [DOI] [PubMed] [Google Scholar]
- 15.Moberley, S. A., J. Holden, D. P. Tatham, and R. M. Andrews. 2008. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2008:CD000422. [DOI] [PubMed] [Google Scholar]
- 16.Mufson, M. A., D. F. Hughey, C. E. Turner, and G. Schiffman. 1991. Revaccination with pneumococcal vaccine of elderly persons 6 years after primary vaccination. Vaccine 9:403-407. [DOI] [PubMed] [Google Scholar]
- 17.Musher, D. M., J. E. Groover, E. A. Graviss, and R. E. Baughn. 1996. The lack of association between aging and postvaccination levels of IgG antibody to capsular polysaccharides of Streptococcus pneumoniae. Clin. Infect. Dis. 22:165-167. [DOI] [PubMed] [Google Scholar]
- 18.Musher, D. M., et al. 1993. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin. Infect. Dis. 17:66-73. [DOI] [PubMed] [Google Scholar]
- 19.Musher, D. M., et al. 2010. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J. Infect. Dis. 201:516-524. [DOI] [PubMed] [Google Scholar]
- 20.Musher, D. M., H. M. Phan, D. A. Watson, and R. E. Baughn. 2000. Antibody to capsular polysaccharide of Streptococcus pneumoniae at the time of hospital admission for pneumococcal pneumonia. J. Infect. Dis. 182:158-167. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien, K. L., M. Hochman, and D. Goldblatt. 2007. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect. Dis. 7:597-606. [DOI] [PubMed] [Google Scholar]
- 22.Pandey, J. P. 2000. Immunoglobulin GM and KM allotypes and vaccine immunity. Vaccine 19:613-617. [DOI] [PubMed] [Google Scholar]
- 23.Ridda, I., et al. 2009. Immunological responses to pneumococcal vaccine in frail older people. Vaccine 27:1628-1636. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Barradas, M. C., et al. 1996. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J. Infect. Dis. 173:1347-1353. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Steiner, S., et al. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 26.Ruben, F. L., and M. Uhrin. 1985. Specific immunoglobulin-class antibody responses in the elderly before and after 14-valent pneumococcal vaccine. J. Infect. Dis. 151:845-849. [DOI] [PubMed] [Google Scholar]
- 27.Sankilampi, U., P. O. Honkanen, A. Bloigu, E. Herva, and M. Leinonen. 1996. Antibody response to pneumococcal capsular polysaccharide vaccine in the elderly. J. Infect. Dis. 173:387-393. [DOI] [PubMed] [Google Scholar]
- 28.Sankilampi, U., P. O. Honkanen, A. Bloigu, and M. Leinonen. 1997. Persistence of antibodies to pneumococcal capsular polysaccharide vaccine in the elderly. J. Infect. Dis. 176:1100-1104. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro, E. D., et al. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 30.Smithson, S. L., N. Srivastava, and M. A. Westerink. 2002. Differential V gene expression detected in the immune response to Streptococcus pneumoniae capsular polysaccharide between elderly and young adults. Hybrid Hybridomics 21:19-24. [DOI] [PubMed] [Google Scholar]
- 31.Stein, K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165(Suppl. 1):S49-S52. [DOI] [PubMed] [Google Scholar]
- 32.Subramaniam, K. S., R. Segal, R. H. Lyles, M. C. Rodriguez-Barradas, and L. A. Pirofski. 2003. Qualitative change in antibody responses of human immunodeficiency virus-infected individuals to pneumococcal capsular polysaccharide vaccination associated with highly active antiretroviral therapy. J. Infect. Dis. 187:758-768. [DOI] [PubMed] [Google Scholar]
- 33.Sun, Y., et al. 1999. Repertoire of human antibodies against the polysaccharide capsule of Streptococcus pneumoniae serotype 6B. Infect. Immun. 67:1172-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torling, J., J. Hedlund, H. B. Konradsen, and A. Ortqvist. 2003. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 22:96-103. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., and B. D. Stollar. 1999. Immunoglobulin VH gene expression in human aging. Clin. Immunol. 93:132-142. [DOI] [PubMed] [Google Scholar]
- 36.Wernette, C. M., et al. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitney, C. G., et al. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 38.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, J., K. R. Lottenbach, S. J. Barenkamp, A. H. Lucas, and D. C. Reason. 2002. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect. Immun. 70:4083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]