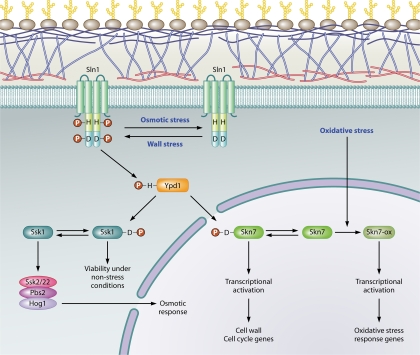
Fig. 1.
The SLN1 phosphorelay in S. cerevisiae. The flow of phosphoryl groups through the SLN1 pathway is shown schematically. Plasma membrane-associated Sln1 kinase is autophosphorylated on the H576 residue under normal growth conditions. Hyperactivation occurs under conditions causing weakening/remodeling of the cell wall (wall stress). Hyperosmolarity and other conditions causing reduced turgor lead to a reduction in Sln1 kinase activity and accumulation of Sln1 in the dephosphorylated form. The phosphoryl group on H576 (H) is transferred to D1144 (D) in the receiver domain of Sln1 and then to H64 of the phosphotransferase, Ypd1, and finally to D554 and D427 in the receiver domains of the cytoplasmic Ssk1 and nuclear Skn7 response regulators, respectively. The unphosphorylated form of Ssk1 interacts with and stimulates the activity of the Ssk2 and Ssk22 MAPKKKs of the HOG1 pathway, while the phosphorylated form of Ssk1 renders it inactive. Phosphorylation of Skn7 leads to activation of a subset of SKN7-dependent genes such as the mannosyltransferase gene, OCH1. However, not all SKN7-dependent genes require aspartyl phosphorylation. For example, SKN7-dependent activation of oxidative stress-responsive genes is independent of the SLN1 pathway.