Abstract
Cerebral malaria (CM) is a primary cause of deaths caused by Plasmodium falciparum in young children in sub-Saharan Africa. Laboratory tests based on early detection of host biomarkers in patient blood would help in the prognosis and differential diagnosis of CM. Using the Plasmodium berghei ANKA murine model of experimental cerebral malaria (ECM), we have identified over 300 putative diagnostic biomarkers of ECM in the circulation by comparing the whole-blood transcriptional profiles of resistant mice (BALB/c) to those of two susceptible strains (C57BL/6 and CBA/CaJ). Our results suggest that the transcriptional profile of whole blood captures the molecular and immunological events associated with the pathogenesis of disease. We find that during ECM, erythropoiesis is dysfunctional, thrombocytopenia is evident, and glycosylation of cell surface components may be modified. Furthermore, analysis of immunity-related genes suggests that slightly distinct mechanisms of immunopathogenesis may operate in susceptible C57BL/6 and CBA/CaJ mice. Furthermore, our data set has allowed us to create a molecular signature of ECM composed of a subset of circulatory markers. Complement component C1q, β-chain, nonspecific cytotoxic cell receptor protein 1, prostate stem cell antigen, DnaJC, member 15, glutathione S-transferase omega-1, and thymidine kinase 1 were overexpressed in blood during the symptomatic phase of ECM, as measured by quantitative real-time PCR analysis. These studies provide the first host transcriptome database that is uniquely altered during the pathogenesis of ECM in blood. A subset of these mediators of ECM warrant validation in P. falciparum-infected young African children as diagnostic markers of CM.
Cerebral malaria (CM) is a primary cause of deaths caused by Plasmodium falciparum, with the majority of cases occurring in young children living in sub-Saharan Africa. CM is characterized by coma, presence of peripheral asexual P. falciparum parasites, and exclusion of other causes of encephalopathy. Early and rapid administration of malaria chemotherapeutic agents (quinine or artemisinins) is currently the only effective treatment for CM. However, even with the best treatment, patients with CM have a case fatality rate of 15 to 20% (29, 37). Novel methods for early prognosis and differential diagnosis are critically needed to reduce the high mortality rate of CM. The need for reliable indicators of clinical disease was first underscored by an autopsy study that demonstrated that the standard clinical case definition of CM is incorrect approximately 25% of the time (38). Recently, clinical studies have shown that the accuracy of a CM diagnosis can be increased by investigation of malarial retinopathy in the differential diagnosis of CM (2, 37). However, additional measures (i) to predict which cases of uncomplicated malaria progress into cerebral malaria and (ii) to discriminate cases of CM from other causes of encephalopathy will be required to improve the clinical outcome of CM.
Cancer prognosis and diagnosis typically rely on the ability to monitor the composition and size of the tumor. However, efforts to establish noninvasive approaches to monitor cancer are currently being explored in the field of oncology. In a study of pancreatic cancer, patients could be discriminated from healthy subjects by a signature of 19 serum proteins (18). In another study, efforts to develop a highly sensitive noninvasive test for colorectal cancer were met with some success by using a panel of five genes detectable in whole blood (16). Likewise, in recent years, some progress has been made in the identification of correlates of CM in blood (22, 26). However, a robust test incorporating a large panel of molecules will likely be required for the accurate prognosis and differential diagnosis of CM. Interestingly, a recent study conducted on a dengue cohort in Brazil demonstrated that microarray analysis of peripheral blood mononuclear cells could be used to design gene expression signatures that accurately predict which cases of acute febrile dengue progress into dengue hemorrhagic fever (28).
Previously, to better understand the pathogenic mechanisms operating during ECM, we used the Plasmodium berghei ANKA murine model of experimental cerebral malaria (ECM), a useful surrogate of human CM (9), to identify over 200 brain-specific biomarkers of ECM by comparing the tissue transcriptional profiles of moribund mice to those of nonmoribund mice and three types of resistant mice (32). In subsequent studies, we assessed the biological relevance of a small subset of these biomarkers and demonstrated that two molecules, CD14 and galectin-3, play a direct role in the pathogenesis of disease (31). In the present study, we have identified circulatory biomarkers of ECM that could be of prognostic/diagnostic value and may suggest novel therapeutic candidates for human CM. We compared the whole-blood gene expression profiles of two susceptible strains of mice (C57BL/6 and CBA/CaJ) with ECM to that of resistant mice (BALB/c). Using this approach, we identified 386 potential biomarkers of ECM (45% upregulated, 55% downregulated) detectable in the blood. Furthermore, our analysis of the gene expression patterns provides novel functional clues regarding the molecular mechanisms that culminate in the pathogenesis of CM and candidate molecules for diagnostic purposes.
MATERIALS AND METHODS
Mice and parasite infections.
Six- to 8-week-old female C57BL/6, CBA/CaJ, and BALB/c mice were purchased from the National Cancer Institute (NCI) breeding facility (Frederick, MD). Parasite infections were performed with an uncloned line of P. berghei ANKA parasites stored in liquid nitrogen as frozen stabilities. Thawed parasites were injected and expanded in donor mice. Experimental mice were then infected by intraperitoneal injection of 106 P. berghei ANKA parasites, and mice were monitored for symptoms of ECM beginning on day 3 postinfection. Thin blood films were prepared for each mouse prior to sample collection, and percentages of parasitemia (calculated as number of parasitized erythrocytes/total erythrocytes × 100) were enumerated by examination of Geimsa-stained blood films.
Measurement of hematocrit.
The hematocrit of whole blood was determined by using the Autocrit Ultra-3 direct reading centrifuge (BD Biosciences, San Jose, CA). Briefly, whole blood was collected in a heparinized capillary tube, sealed with Seal-Ease tube sealer, and then centrifuged at a fixed speed of 11,700 rpm for 3 min. The hematocrit was then determined with a centrifuge-scale plate.
Preparation of whole-blood RNA.
Whole blood was collected directly into a tube preloaded with RNAlater for immediate stabilization of RNA, mixed thoroughly, and stored for no longer than 10 days at −20°C until use. RNA was prepared using the mouse RiboPure blood isolation kit (Ambion, Inc., Austin, TX). Briefly, supernatant was removed from thawed samples after centrifugation at high speed. RNA was then isolated by lysis of sedimented blood with a guanidinium-based solution, extraction with acid-phenol-chloroform, and purification with a glass fiber filter cartridge. RNA loaded onto the filter cartridge was then washed twice, eluted in nuclease-free water, and concentrated by ultrafiltration with a Vivaspin 500 column (Sartorius Stedim Biotech, Goettingen, Germany) to approximately 3 μg/μl for subsequent direct labeling.
Microarray and gene expression analysis.
Peripheral blood global gene expression profiles of two susceptible strains of mice (C57BL/6 and CBA/CaJ) were compared to those of resistant BALB/c mice. Expression profiles were determined from RNA isolated from five mice per group, and importantly, RNA samples isolated from susceptible and resistant mice were matched not only by day of blood collection (when susceptible mice exhibited symptoms of ECM) but also by percent parasitemia (since parasite RNA is likely a significant component of infected peripheral blood total RNA). Furthermore, microarray studies were performed using samples collected on day 6 or 7 postinfection in order to minimize variations that may arise due to the stage of infection. Synthesis, incorporation of label, and purification of cDNA were performed as previously described (30, 32), and then labeled cDNAs from matched samples (approximately 30 pmol each) were combined and hybridized to a custom-designed murine oligonucleotide chip containing 37,500 probes (Agilent Technologies, Santa Clara, CA).
Only spots with valid measurements in 80% of the arrays were considered in our analysis. Differentially expressed genes were assessed by two different criteria: (i) fold change of ≥2 in 80% (4/5) in each of the susceptible strains; (ii) differential expression detected by the significance analysis of microarray (SAM) method (one-class mode; false discovery rate (FDR) = 0.011) (39). In the latter case, the genetic background of the susceptible mice was disregarded, and SAM was performed in the one-class mode using 10 data points. SAM was also used to find genes with differential expression between the susceptible strains (two-class unpaired mode; FDR = 0.032). The list of differentially expressed probes was mapped to Entrez Gene (27), and a single probe was manually selected for each gene based on fold change and standard deviation. Differentially expressed probes that did not map to an Entrez Gene ID or UniGene Gene were excluded from our analysis. Gene Ontology (GO) enrichment analysis was performed using FuncAssociate (3) (1,000 permutations and corrected P value threshold of 0.05).
Real-time PCR.
RNA was first subjected to two rounds of rigorous DNase treatment with 6 U of Turbo DNA-free (Ambion, Inc., Austin, TX) at 37°C for 30 min, and absence of DNA contamination was verified by gel electrophoresis analysis of PCR product amplified from RNA template with primers specific for the mouse β-actin gene. To increase the sensitivity of the assay, globin mRNA, which represents as much as 70% of the total mRNA population, was removed from whole-blood RNA by using GLOBINclear-mouse/rat (Ambion, Inc., Austin, TX). cDNA was then synthesized from 1 μg of enriched RNA at 42°C for 30 min in a 20-μl reaction volume containing iScript reverse transcriptase, random primers, deoxynucleoside triphosphates (dNTPs), and magnesium chloride (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time PCR (qRT-PCR) was performed in a 25-μl reaction volume containing 2 μl of cDNA, 12.5 μl iQ SYBR green supermix (Bio-Rad Laboratories, Hercules, CA), and 10 μM gene-specific primers. Amplification and detection of specific product were performed with the iCycler iQ5 real-time PCR instrument (Bio-Rad Laboratories, Hercules, CA) with the following cycle profile: 1 cycle at 95°C for 10 min; 5 cycles with 1 cycle consisting of 20 s of denaturation at 94°C, 15 s of annealing at 48°C, and a 30-s extension at 68°C; and 40 cycles with 1 cycle consisting of 20 s of denaturation at 94°C, 15 s of annealing at 57 or 60°C, and a 30-s extension at 68°C. A standard curve derived from the PCR products of 10-fold serial dilutions of plasmid containing a mouse β-actin gene fragment was used to determine the absolute concentration of RNA. Real-time PCR was performed on three mice per group twice in separate reactions.
RESULTS
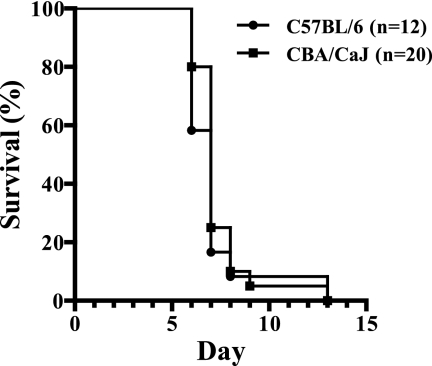
Two distinct susceptible strains (C57BL/6 and CBA/CaJ) and one resistant strain (BALB/c) of mice were used in our study. We selected a study design that included two susceptible strains of mice to determine the effects of host genetic background on the expression of ECM in mice. The mortality rates from ECM in C57BL/6 and CBA/CaJ mice were 91.7% (11 of 12 mice) and 95.0% (19 of 20 mice), respectively, with the majority of mice developing symptoms of ECM on day 6 or 7 after infection (Fig. 1). In contrast, although BALB/c mice developed systemic parasitemia and anemia, they did not exhibit symptoms of ECM. Interestingly, although both susceptible mouse strains had similar mortality rates, we observed noticeable differences in the clinical symptoms of ECM. We found that in C57BL/6 mice, progression of disease was gradual and the majority of mice displayed symptoms of irreversible coma during the terminal phase of disease, whereas in CBA/CaJ mice, the onset of disease was comparatively more sudden and symptoms of rigorous seizures were a more prominent feature of disease. In order to investigate this observation at the molecular level, we separately analyzed similarities and differences in gene expression during ECM between the two susceptible strains.
FIG. 1.
Susceptibility of CBA/CaJ and C57BL/6 mice to ECM. Eleven of 12 (91.7%) C57BL/6 mice and 19 of 20 (95%) CBA/CaJ mice developed symptoms of ECM by day 9 postinfection.
Identification of shared molecular features of susceptible strains during experimental cerebral malaria.
To identify the common denominator in the molecular signatures of ECM in two susceptible strains exhibiting slightly different clinical symptoms, we compared the global expression profiles in peripheral blood of the C57BL/6 and CBA/CaJ strains of mice and clustered them as a single class versus those of the resistant BALB/c mice. A gene was considered differentially expressed when meeting at least one of the following criteria: (i) 2-fold change in at least 80% of the samples combining both susceptible strains; or (ii) differential expression detected by the SAM method (see Materials and Methods for details). Using the criteria outlined above, we found a total of 1,379 differentially expressed genes; of these 617 were upregulated and 762 were downregulated (Δ= 1.88; FDR = 0.011) (see Tables S1, S2, and S3 in the supplemental material). We then explored differentially expressed genes by checking for enrichment of Gene Ontology (GO) terms (see Materials and Methods for details). A major outcome of this analysis was that certain potential chromatin-related functions were significantly upregulated, whereas those related to heme biosynthesis were significantly downregulated. To further investigate these data, we performed a more in-depth analysis of the genes showing the most drastic expression changes (>4-fold): a set of 176 upregulated and 210 downregulated genes. Using sequence profile searches, analysis of literature, and protein-protein/genetic interactions, we functionally classified these genes to get a better picture of the transcriptional landscape of the peripheral blood in ECM.
Evidence for dysfunctional erythropoiesis during ECM.
Interestingly, we found evidence for pervasive erythroid dysfunction in the susceptible compared to resistant strains of mice, with at least 23 genes related to erythropoiesis showing greater than 4-fold downregulation in mice exhibiting symptoms of ECM. As the initial GO analysis revealed a downregulation of heme biosynthesis genes in the susceptible mice, we analyzed this issue in greater detail. Genes encoding three key enzymes at different points in the heme biosynthesis pathway, namely, aminolevulinic acid synthase, hydroxymethylbilane synthase, and ferrochelatase were all downregulated greater than 8-fold on average. Concomitantly, the Hbb-b1 and Hbb-b2 hemoglobin genes and the alpha hemoglobin-stabilizing protein genes were also comparably downregulated. Beyond heme biosynthesis, several structural and metabolic proteins specific to the erythroid lineage were also similarly depressed in regulation. These included genes that encode red blood cell (RBC) cytoskeletal proteins such as spectrin alpha 1, ankyrin 1, erythroid protein bands 4.1 and 4.2, and adducin 2 (Table 1) and cell surface components such as the sialoglycoprotein glycophorin A (notably, a receptor for P. falciparum erythrocyte binding antigen 175) (5), Kell blood group protein, and the Rh blood group D antigen. The strong downregulation of several genes related to glutathione metabolism (Table 1) and genes encoding proteins such as Isca1 (involved in iron-sulfur cluster assembly), 2,3-bisphosphoglycerate mutase, and ornithine decarboxylase might also be related to the transcriptional depression of genes associated with erythrocyte metabolic pathways. Also downregulated were potential erythrocyte regulators such hemogen and a possible trafficking component, α-synuclein. Interestingly, at least five of these genes recovered as downregulated, namely, SNCA, ALAS2, FECH, AHSP, and HEMGN, are known to be targets of the major erythroid transcription factor GATA-1. GATA-1 was not found to be significantly downregulated in the susceptible mice, but its functional partners E2F2 and Dp2, required for terminal cell division and maturation during erythropoiesis (19), were 5- and 7-fold downregulated, respectively. Hence, we speculate that the depressed erythroid development might be a consequence of the depressed expression of this key transcription factor.
TABLE 1.
Circulatory biomarkers of ECM categorized by function
| Gene transcript | Function/description | Fold change in expression |
|---|---|---|
| Erythropoiesis-related genes | ||
| ALAS2 | Aminolevulinic acid synthase 2, erythroid | −14.2 |
| FECH | Ferrochelatase | −17.2 |
| AHSP | Alpha hemoglobin stabilizing protein | −11.2 |
| HBB-B2 | Hemoglobin, beta adult major chain | −11.6 |
| HMBS | Hydroxymethylbilane synthase | −8.4 |
| SNCA | Synuclein, alpha | −8.2 |
| HBB-B1 | Hemoglobin, beta adult major chain | −7.7 |
| SPNA1 | Spectrin alpha 1 | −6.8 |
| ANK1 | Ankyrin 1, erythroid | −4.1 |
| EPB4.1 | Erythrocyte protein band 4.1 | −6.4 |
| EPB4.2 | Erythrocyte protein band 4.2 | −7.6 |
| ADD2 | Adducin 2 | −5.4 |
| KEL | Kell blood group protein | −5.1 |
| RHD | Rh blood group, D antigen | −7.0 |
| HEMGN | Hemogen | −4.3 |
| Glutathione metabolism-related genes | ||
| HAGH | Hydroxyacyl glutathione hydrolase | −13.8 |
| GCLM | Glutamate-cysteine ligase, modifier subunit | −7.2 |
| GLRX5 | Glutaredoxin 5 homolog (Saccharomyces cerevisiae) | −5.2 |
| TXNRD2 | Thioredoxin reductase 2 | −4.4 |
| GPX4 | Glutathione peroxidase 4 | −4.0 |
| Platelet and clotting-related genes | ||
| F5 | Coagulation factor V | −5.7 |
| F13A1 | Coagulation factor XIII A1 subunit | −5.3 |
| VWF | von Willebrand factor | −4.9 |
| PDGFA | Platelet-derived growth factor alpha | −4.6 |
| ANGPT1 | Angiopoietin 1 | −4.2 |
| Cell surface glycosylation-related genes | ||
| GYPA | Sialoglycoprotein glycophorin A | −22.6 |
| CMAS | Cytidine monophospho-N-acetylneuraminic acid synthetase | −6.7 |
| ST3GAL5 | ST3 beta-galactoside alpha-2,3-sialyltransferase 5 | −4.4 |
| ST3GAL2 | ST3 beta-galactoside alpha-2,3-sialyltransferase 2 | −4.2 |
| HEXA | Hexosaminidase A | −4.2 |
| HS3ST3B1 | Heparan sulfate (glucosamine) 3-O-sulfotransferase 3B1 | 4.7 |
| Immune response-related genes | ||
| NPY | Neuropeptide Y | −292.8 |
| H2-EA | Histocompatibility 2, class II antigen E alpha | −53.1 |
| H2-AB1 | Histocompatibility 2, class II antigen A, beta 1 | −11.4 |
| ISG20 | Interferon-stimulated protein | −6.8 |
| DEFA1 | Defensin, alpha 1 | −5.1 |
| IIGP1 | Interferon-inducible GTPase 1 | −4.2 |
| OAS1E | 2′-5′ Oligoadenylate synthetase 1E | −5.7 |
| OAS3 | 2′-5′ Oligoadenylate synthetase 3 | −4.1 |
| CXCL2 | Chemokine (C-X-C motif) ligand 2 | 32.3 |
| CX3CR1 | Chemokine (C-X3-C) receptor 1 | 7.5 |
| MAL | Myelin and lymphocyte protein, T-cell differentiation protein | 10.1 |
| NCCRP1 | Nonspecific cytotoxic cell receptor protein 1 | 6.1 |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 | 6.3 |
| KLRA8 | Killer cell lectin-like receptor, subfamily A, member 8 | 6.8 |
| KLRA15 | Killer cell lectin-like receptor, subfamily A, member 15 | 5.8 |
| Transcription and chromatin structure-related genes | ||
| TFDP2 | Transcription factor Dp 2 | −6.6 |
| STAT1 | Signal transducer and activator of transcription 1 | −6.4 |
| E2F2 | E2F transcription factor 2 | −5.3 |
| SPIB | ETS domain transcription factor SpiB | −4.9 |
| RBPJL | Recombination signal binding protein for immunoglobulin kappa J region-like | 17.6 |
| UBN2 | Ubinuclein 2 | 15.9 |
| HOPX | HOP homeobox | 4.8 |
| Ubiquitin system-related genes | ||
| TRIM10 | Tripartite motif-containing 10 | −84.4 |
| UBE2L6 | Ubiquitin-conjugating enzyme E2L 6 | −10.0 |
| RNF10 | Ring finger protein 10 | −8.7 |
| FBXO25 | F-box protein 25 | −8.5 |
| USP32 | Ubiquitin specific peptidase 32 | −8.2 |
| MARCH2 | Membrane-associated ring finger (C3HC4) 2; E3 ligase | −7.1 |
| MARCH8 | Membrane-associated ring finger (C3HC4) 8; E3 ligase | −6.2 |
| FBXO9 | F-box protein 9 | −5.6 |
| UBE2R2 | Ubiquitin-conjugating enzyme E2R 2 | −4.6 |
| RAD23A | RAD23a homolog (S. cerevisiae) | −4.1 |
| UBAC1 | Ubiquitin-associated domain containing 1 | −4.1 |
| ATG4A | Autophagy-related 4A (yeast) | −7.4 |
| Apoptosis-related genes | ||
| BCL2A1A | B-cell leukemia/lymphoma 2-related protein A1a | 4.0 |
| Cytoskeletal keratin genes | ||
| KRT12 | Keratin 12 | 6.2 |
| KRT23 | Keratin 23 | 20.0 |
| KRT7 | Keratin 7 | 18.5 |
| KRT6B | Keratin 6B | 17.2 |
| KRT13 | Keratin 13 | 15.7 |
| KRT19 | Keratin 19 | 8.4 |
| Metabolism-related genes | ||
| ISCA1 | Iron-sulfur cluster assembly 1 homolog | −5.9 |
| BPGM | 2,3-Bisphosphoglycerate mutase | −15.4 |
| ODC1 | Ornithine decarboxylase | −5.7 |
We next wanted to determine a possible relationship between P. berghei ANKA infection-induced anemia and progression into ECM in susceptible and resistant mice. We first measured the hematocrit in uninfected mice to ensure that there were no strain-specific differences in basal hematocrit levels. The hematocrits were 47.4 ± 1.4 (C57BL/6) and 44.2 ± 1.9 (CBA/CaJ) in susceptible mice and 48.7 ± 1.2 (BALB/c) in resistant mice. We then measured the hematocrit of infected mice during the symptomatic phase (day 6) of disease. Susceptible mice with ECM had hematocrits of 47.3 ± 0.6 (C57BL/6) and 47.8 ± 1.5 (CBA/CaJ). The hematocrit of resistant BALB/c mice was similar (47.1 ± 1.1), indicating a lack of association between anemia and expression of ECM in mice. These results also suggest dyserythropoiesis observed by microarray in mice with ECM does not result in altered hematocrit as measured on day 6 postinfection. These discordant results may be due to the likelihood that young erythrocytes are only a small fraction of total erythrocytes in blood.
Downregulation of platelet- and clotting-related genes in ECM.
We observed that among the highly downregulated genes, there were genes related to platelet and blood-clotting functions such as those encoding von Willebrand factor, coagulation factor XIII A1 subunit, platelet-derived growth factor alpha, and coagulation factor V. Additionally, angiopoietin 1, which stabilizes microvessels against leakage (15) and is required for the recovery of the brain from hypoxia (33), was also >4-fold downregulated. Taken together, these results suggest that the susceptible mice are likely to suffer from greater leakage via their microvessels along with reduced clotting leading to a propensity for hemorrhaging. In light of this, potential therapeutic regimens which specifically target restoration of the angiopoietin balance and the depletion of coagulation factors should be further explored.
Possible alterations in the signature of cell surface glycosylation in ECM.
Our data also presented evidence for several distinct enzymes involved in modification of cell-surface polysaccharides to be strongly differentially regulated between susceptible and resistant strains. Of the downregulated genes are those coding for the enzymes ST3 β-galactoside α-2,3-sialyltransferase 2 and 5, which are involved in the synthesis of glycosphingolipids such as the gangliosides GM3 and GM4 (7). Consistent with this, the cytidine monophospho-N-acetylneuraminic acid synthetase, which synthesizes a potential substrate for the above glycosyltransferases, is also concomitantly downregulated in ECM. Another enzyme, hexosaminidase A, involved in the degradation of gangliosides such as GM2, is also downregulated. Cell-surface gangliosides have previously been observed to be major targets for interaction with pathogen proteins in the process of invasion of eukaryotic host cells by diverse pathogens (21). These transcriptional changes could in effect result in the reduced presentation of a particular type of ganglioside on the host cell surface and thereby modulate a specific host-parasite interaction. Hence, it is conceivable that their transcriptional regulation might be a defensive response to regulate host-parasite interaction. In contrast to these genes, the Hs3st3b1 gene, involved in sulfation of glucosamine 3-OH in cell-surface heparan sulfate, is upregulated strongly. We have previously observed that the polysaccharide-binding protein galectin-3 has a notable role in cerebral malaria progression, but it does not appear to directly bind any pathogen moiety (31). Given this observation, it is also possible that the alterations in host cell-surface polysaccharides by downregulation of certain pathways and upregulation of others might have a role in regulating endogenous cell adhesion reactions. Future studies that could directly assess differences in cell-surface glycosylation may allow investigation of its utility as a potential prognostic marker in cerebral malaria.
Alteration in the immune response in relation to ECM.
Not surprisingly, the functional class showing the largest number of genes with more than 4-fold-altered expression in susceptible mice relative to the resistant mice is that pertaining to the immune response. This group includes 65 genes, with 26 genes downregulated and 39 genes upregulated. We observed that the ETS domain transcription factor SpiB, which is a key regulator of the development of a specific class of immune cells, the plasmacytoid dendritic cells (natural interferon-producing cells) (35), is downregulated approximately 5-fold in ECM-susceptible mice. Consistent with this, we observed a major downregulation of genes associated with such cells or those genes induced by the cytokines secreted by these cells (e.g., the STAT1, IIGP1, DEFA1, ISG20, OAS1E, and OAS3 genes) (Table 1). The primary role of plasmacytoid dendritic cells is believed to be in innate immunity against viruses. deWalick et al. have shown that conventional but not plasmacytoid dendritic cells play a key role in the pathogenesis of ECM; depletion of conventional dendritic cells early in P. berghei ANKA infection with B6.CD11c-DTR transgenic mice prevented symptoms of ECM, whereas depletion of plasmacytoid dendritic cells by using the 120G8 monoclonal antibody (MAb) had no effect on susceptibility to ECM (10). Given that SpiB promotes the development of these dendritic cells at the expense of T, B, and NK cells, it is conceivable that downregulation of this transcription factor might have a role in allowing alternative developmental programs for blood cells which are required for the parasite response. Indeed, several genes of cytotoxic T and NK cell receptors (e.g., the Klra15, Nccrp1, Ctla4, Klra8, and MAL genes) are upregulated in our data set. Nevertheless, it is conceivable that this observation provides a means of distinguishing an infection that results in ECM.
Another novel observation emerging from our data set is extraordinary downregulation (292-fold on average) of the neuropeptide Y gene (NPY). Npy has been shown to be secreted in the peripheral blood by macrophages and B cells and shown to regulate many aspects of the immune response, such as macrophage behavior, antibody secretion by B cells, T-cell proliferation, and uptake of antigens by dendritic cells (43). Given this pervasive role, the drastic downregulation of Npy could potentially alter the landscape of the immune response in a major way. Hence, further investigation of the role of Npy in ECM pathogenesis is warranted as it could offer both a potential therapeutic as well as diagnostic tool. Among the other secreted signaling molecules showing drastic differences in expression was the gene encoding the chemokine Cxcl2 (32-fold upregulated in susceptible mice). This chemokine plays a major role as a neutrophil attractant and has been implicated in certain inflammatory diseases (14). It would be of interest to investigate if this molecule might have a role in the progression of ECM, especially given its elevated expression. Likewise, the receptor of the chemokine fractalkine, CX3CR1, is also overexpressed in susceptible mice (7.5-fold). This chemokine receptor has been previously implicated in endothelial damage in other infectious diseases such as cytomegalovirus infection (4). In light of this, it would be of interest to investigate if CX3CR1 plays a role in facilitating the hemorrhaging observed in CM. The above observations suggest that several distinct immune pathways that have not previously been considered in the context of malarial pathogenesis could be worth investigating in future studies.
Differential expression of transcription and chromatin structure-related genes.
We observed that 27 genes (15 downregulated and 12 upregulated) encoding proteins with a definitive role in transcription regulation or chromatin organization were differentially expressed (>4-fold) in the susceptible versus the resistant mice. In addition to some of the transcription factors that were discussed above, we identified certain others which might play important roles in different blood cells. For example, Rbpjl, a transcription factor of the CSL family, is upregulated 17.5-fold in the susceptible mice. A role for this transcription factor, which functions downstream of the Notch signaling cascade, in hemal development has not been previously uncovered. Given its dramatic expression pattern, we suggest that it might be involved in an asymmetric cell division or differentiation of immune cells downstream of an as yet unclear wing of the Notch pathway. Similarly, the homeodomain transcription factor, Hopx, has not been implicated in a blood cell-related role. Its emergence as a prominently upregulated gene product in our study might implicate it as an as yet undiscovered transcription factor in a hemal differentiation process. Several chromatin proteins were found to be systematically downregulated (Table 1), suggesting that these chromatin-level alterations might be linked to the differential gene expression patterns observed in our study. However, the ubinuclein-2 (Ubn2) gene was notably upregulated (16-fold). We had recently indentified this protein to be a novel histone chaperone that functions in conjunction with the Hir complex (1). Given its detection in our experiments, it would be of interest to investigate if Ubn2 might have a specific role in the differential expression of genes during the immune response or hemocyte development via the reorganization of nucleosomes.
Other miscellaneous genes of interest showing differential expression.
While the above functional categories contain most of the genes of interest showing marked differential expression, we found some other genes with unusual expression differences which might have functional relevance to ECM pathogenesis. Interestingly, 13 of the 16 genes related to the ubiquitin system that showed greater than 4-fold expression change were downregulated. This significantly biased downregulation was seen across the Ub pathway, including genes coding for at least 5 E3 ligases, 2 E2 ligases and two debuiquitinating enzymes (see the supplemental material). Of particular interest are the March2 and March8 E3 ligases that have been recently implicated in the Ub-dependent trafficking of major histocompatibility complex (MHC) class II molecules into secretary vesicles (11). Their downregulation is consistent with the concomitant downregulation of various class II MHC proteins such as H2-Ab1 (11-fold) and H2-Ea (53-fold) (Table 1). In light of this, it would be worthwhile for future investigations on CM pathogenesis to consider the role of the downregulation of multiple Ub system genes in the context of depressed class II MHC expression. Also of interest was the upregulation of the Bcl2 paralog, Bcl2a1a, which has been implicated in the survival of double-positive thymocytes (41). It remains to be seen if the overexpression of this antiapoptotic factor has a significant role in altering the immune cell development between susceptible and resistant strains. Notably, five distinct cytoskeletal keratin genes (Table 1) are between 11- and 20-fold overexpressed in the susceptible strains. The cell types in which these might be overexpressed are unknown, but they could potentially serve as a diagnostic marker.
Identification of the genes differentially expressed between susceptible strains during the ECM.
We also compared the susceptible strains against each other to find genes with differential expression between two genetically different backgrounds (see the supplemental material). In this analysis, the BALB/c (ECM-resistant) strain was considered a common reference to find altered expression between the susceptible strains. We found significantly altered expression in 93 genes. There was a remarkable overrepresentation of immune-related genes, especially those related to the MHC (see the supplemental material). Twenty-one of these 93 genes were also found to be differentially expressed when comparing susceptible versus resistant strains (as described above). However, the vast majority of the immune-related genes with significant variation between strains were not recovered identified in the above analysis treating the two susceptible strains as one class. This implies that above-identified genes related to immunity are likely to serve as useful genetic markers for ECM diagnosis, irrespective of the strain background. It also suggests that the divergent regulation of several immune response genes might result in distinct immunity landscapes in the two susceptible strains, which might have a role in the symptomatic differences observed between them.
Interestingly, while the gene that encodes CD8 antigen, beta chain 1 (CD8β1) is significantly upregulated (3.9 ± 0.5; P < 0.0005) in moribund C57BL/6 mice compared to resistant BALB/c mice, it is markedly downregulated (−7.5 ± 1.2; P < 0.0003) in the peripheral blood of moribund CBA/CaJ mice. Of considerable interest are the diametrically opposite expression patterns of certain NK cell receptors between the two susceptible strains: Klra4, Klra21, and Klra18 are all clearly upregulated in C57BL/6 mice, while being unchanged or mildly downregulated in CBA/CaJ mice. In contrast, Ncr1 is downregulated in only C57BL/6 mice. The exact role of these NK cell receptors in malaria remains unclear; however, the fact that some of them are similarly upregulated in both susceptible strains, whereas others are overexpressed in only one strain, might suggest some as yet unclear role for them in parasite response.
Of note is the strain-specific upregulation of genes encoding two distinct chemokines, Ccl12 and Cxcl11, in the CBA/CaJ mice. Ccl12 is a chemoattractant that recruits certain types of regulatory T cells to sites of inflammation. In contrast, Cxcl11 has been previously implicated in a number of human disease conditions. Of particular interest is its role in neuroborreliosis, where it has been implicated as a chemoattractant for CD4+ T cells in the cerebrospinal fluid (CSF) (24). It is possible that it could play a comparable role in the aspects of ECM pathogenesis specific to the CBA/CaJ mice.
Validation of putative biomarkers by quantitative real-time PCR.
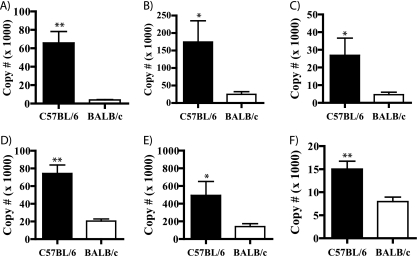
Finally, we wanted to develop a prototype test for the diagnosis of CM based on the transcriptional analysis of host biomarkers in blood. To accomplish this, a panel of genes that were strongly overexpressed (by at least 4-fold) during ECM and functionally diverse was selected for validation by quantitative real-time PCR comparing C57BL/6 (n = 3) versus BALB/c (n = 3) peripheral blood RNA. Only upregulated (not downregulated) genes were chosen because it is easier to measure the upregulation of expression. Furthermore, a functionally diverse panel of genes associated with nonoverlapping pathways was selected to create a molecular signature unique to the disease state of ECM. Complement component C1q, β chain (C1qb) was overexpressed by 17.4 ± 1.3-fold in C57BL/6 (65,800 ± 12,370) versus BALB/c (3,873 ± 430) mice (P = 0.0075) (Fig. 2 A), nonspecific cytotoxic cell receptor protein 1 (nccrp1) by 7.1 ± 0.05-fold in C57BL/6 (174,400 ± 60,980) versus BALB/c (26,690 ± 7,707) mice (P = 0.0716) (Fig. 2B), prostate stem cell antigen (psca) by 5.7 ± 0.3-fold in C57BL/6 (26,920 ± 9,685) versus BALB/c (4,632 ± 1,386) mice (P = 0.0850) (Fig. 2C), DnaJC, member 15 (DnaJC15) by 3.7 ± 0.04-fold in C57BL/6 (74,300 ± 9,607) versus BALB/c (20,400 ± 2,471) mice (P = 0.0056) (Fig. 2D), glutathione S-transferase omega-1 (gsto1) by 3.6 ± 0.5-fold in C57BL/6 (494,200 ± 157,700) versus BALB/c (141,100 ± 32,970) mice (P = 0.0935) (Fig. 2E), and thymidine kinase 1 (tk1) by 1.89 ± 0.005-fold in C57BL/6 (15,020 ± 1,707) versus BALB/c (7,958 ± 983) mice (P = 0.0231) (Fig. 2F). In summary, three of the selected genes (coding for c1qb, DnaJC15, and tk1) had enhanced levels of expression during ECM that were statistically significant (as defined by a P value of <0.05), while the other three genes (coding for nccrp1, psca, and gsto1) had levels of expression that approached statistical significance (as defined by a P value of <0.1).
FIG. 2.
Circulatory biomarkers of ECM as detected by quantitative real-time PCR (qRT-PCR). The expression of a subset of murine circulatory biomarkers of ECM was measured by comparative qRT-PCR in susceptible C57BL/6 mice (n = 3) versus resistant BALB/c mice (n = 3) during the ECM phase of P. berghei ANKA infection. Shown is expression of complement component C1q, β-chain (A); nonspecific cytotoxic cell receptor protein 1 (B); prostate stem cell antigen (C); DnaJC, member 15 (D); (E) glutathione S-transferase omega-1 (E); and thymidine kinase 1 (F). *, P ≤ 0.1; **, P ≤ 0.05.
DISCUSSION
In this study, we identified over 300 potential biomarkers of ECM detectable in the circulation by comparing the whole-blood transcriptional profiles of resistant BALB/c mice to those of two susceptible strains of mice (C57BL/6 and CBA/CaJ) during the symptomatic phase of disease. While the major objective of this study was to identify biomarkers that may be of prognostic/diagnostic value, we note that the transcriptional profile of the peripheral blood captures the molecular and immunological events occurring in the brain and spleen during ECM. To our knowledge, this is the first study that measured the transcription profile of whole blood during the ECM phase in mice; previous studies were performed with brain and spleen samples.
Several important clinical and biological observations were made in this study. We noted that the clinical features of ECM are slightly different between C57BL/6 and CBA/CaJ mice. While C57BL/6 mice slowly progress into a state of coma, CBA/CaJ mice experience a more sudden onset of disease with the obvious signs of seizure prior to coma. Based on the subtle genetic differences, it is reasonable to argue that the distinct clinical features induced by P. berghei ANKA infection in these two inbred mouse strains are regulated by their host genetic factors. It is important to note that the spectrum of clinical features seen in young African children experiencing CM is also known to vary greatly, which is generally attributed to host genetics. Our study has allowed us to discern some of the host factors that may be responsible for these various clinical features observed during ECM. We also determined if anemia correlated with the pathogenesis of ECM by comparing the hematocrits of susceptible mice (C57BL/6 and CBA/CaJ) exhibiting symptoms of ECM to that of resistant BALB/c mice. We found that on day 6 postinfection, all three strains of mice had a similar hematocrit, suggesting that ECM occurs independently of anemia.
Although there was no significant difference in the hematocrits in moribund C57BL/6 and CBA/CaJ versus resistant BALB/c mice, our microarray data suggest that erythropoiesis is comparatively dysfunctional during ECM, with at least 23 genes related to erythropoiesis downregulated by greater than 4-fold in moribund C57BL/6 and CBA/CaJ mice. Erythropoiesis, the differentiation of hematopoietic stem cells into mature red blood cells, is characterized by the extrusion of nuclei from orthochromatic erythroblasts to form enucleated reticulocytes and subsequent mature red blood cells (17). Previously, we reported simultaneous downregulation of transcription but upregulation of translation of the hemoglobin-α gene in brain tissue of mice with ECM (32). Based on these results, we hypothesized that low levels of hemoglobin-α RNA were a result of a paucity of (nucleated) immature erythrocytes within the brain due to decreased production of erythrocytes, while elevations in hemoglobin-α protein were caused by an abundance of mature erythrocytes due to excess hemorrhaging within the brain. The consistent transcriptional repression of genes associated with erythroid differentiation observed in the present study may reflect a lack of young nucleated cells of the erythroid lineage in the peripheral blood and suggests the importance of suppressed erythropoiesis during ECM. However, we want to emphasize that since this study included only one ECM-resistant strain of mice, it is possible that robust infection-induced erythropoiesis may be a feature of the BALB/c strain of mice rather than a phenotype unique to ECM-resistant mice. Future studies on additional strains of ECM-resistant mice will be needed to fully address this question.
In a previous study, transcriptional analysis of uninfected versus P. berghei ANKA-infected C57BL/6 mice prior to the onset of symptoms demonstrated suppressed erythropoiesis in the bone marrow and spleen of mice on days 1, 3, and 5 of infection that translated into reduced levels of peripheral reticulocytes (36). These results indicate that dysfunctional erythropoiesis may occur early in the pathogenesis of ECM. An earlier study that examined bone marrow aspirates of nine patients with cerebral malaria in Thailand suggested that dyserythropoiesis may also be a feature of human CM (44). In this study, microscopic analysis of the bone marrow aspirates presented evidence of morphological abnormalities of erythroblasts that the authors speculated could be caused by local release of malaria toxin, hypoxia, or disturbed macrophage function induced by concomitant cytoadherence of parasitized erythrocytes to endothelial cells in the bone marrow sinusoids. Indeed, recent in vitro studies indicate that hemozoin can directly inhibit erythropoiesis by induction of apoptosis of erythroid precursors, and macrophages actually reduce this inhibitory effect of hemozoin on erythroid development by preventing contact between hemozoin and erythroid cells (23).
Interestingly, murine studies have shown that treatment of mice with erythropoietin reduces mortality from ECM (20, 45). Although the primary function of erythropoietin is regulation of erythrocyte production, these studies have focused on its neuroprotective capacity to prevent hypoxia and apoptosis within the brain. However, a strong association between high levels of plasma but not CSF erythropoietin and a reduced risk of neurological sequelae and death was recently reported in African children with CM (6). Our microarray data suggest that suppressed erythropoiesis does not alter the hematocrit during ECM. These results may be explained by an earlier observation showing that reticulocytes are only a small fraction of total erythrocytes during a P. berghei ANKA infection (36). However, it is plausible that the absence of young erythrocytes may contribute to decreased brain oxygenation, which may augment symptoms and potentially result in neurological sequelae. Future studies will be needed to determine whether dysfunctional erythropoiesis during CM has a causative role in the pathogenesis of disease.
We also found that several genes related to platelet and clotting functions were significantly downregulated during ECM. Numerous studies have implicated a role for platelets in the pathogenesis of ECM (40), and recent studies have examined the role of platelets in human CM. In a study of Malawian children, platelet accumulation in the microvessels of brain tissue was found to be significantly greater in patients with CM than those with severe malaria anemia or nonmalarial encephalopathy (12). In addition, all Malawian pediatric patients with CM had low platelet counts with the degree of thrombocytopenia significantly correlating with parasitemia (42). Indeed, previous clinical studies have indicated that retinal hemorrhaging (a possible indicator of thrombocytopenia) is significantly associated with the severity of CM in humans (25), and malaria retinopathy is currently being explored as a promising tool for the differential diagnosis of CM (2, 37).
Interestingly, CD8β1 is significantly upregulated (3.9 ± 0.5; P < 0.0005) in moribund C57BL/6 mice but markedly downregulated (−7.5 ± 1.2; P < 0.0003) in moribund CBA/CaJ mice compared to resistant BALB/c mice. This suggests that the CD8+ T-cell population produced during a lethal P. berghei ANKA infection that culminates in ECM may differ phenotypically in susceptible C57BL/6 and CBA/CaJ strains of mice. Alternatively, it is possible that absence of CD8β1 in the circulation may result from preferential sequestration of CD8β1+ T cells within the brain by CBA/CaJ mice. Previously, Randall et al. reported that CD8β1 is significantly upregulated in brain tissue of moribund C57BL/6 but not CBA P. berghei ANKA-infected mice (34). In murine studies conducted by Grau et al. in the early 1980s, depletion of CD8+ T cells with an antibody that targets CD8β1 did not prevent the onset of ECM in P. berghei ANKA-infected CBA mice (13). However, it was recently shown that anti-CD8α successfully inhibits the progression of ECM in CBA mice (34). Together, these studies indicate that pathogenic CD8+ T cells may differ phenotypically among susceptible strains of mice.
The primary objective of this study was to identify biomarkers detectable in the circulation that could be used for the prognosis and differential diagnosis of CM. Efforts are under way to identify host biomarker-based correlates of CM. For example, previous studies have assessed the prognostic and diagnostic potential of angiopoietin 1, a marker of the resting endothelium, and angiopoietin 2, a marker of endothelial activation, for CM (26, 8). In a study of Ugandan children and Thai adults, Lovegrove et al. demonstrated that angiopoietin-1 levels are decreased and angiopoietin-2 levels are increased in CM versus uncomplicated malaria and healthy controls and the angiopoietin 2/angiopoietin 1 ratio was useful in predicting survival in African children with CM.
Among the greater than 300 circulatory biomarkers of ECM identified by microarray in our study, we selected a subset of biomarkers for further evaluation by quantitative real-time PCR (qRT-PCR). Differential expression of six molecules (c1qb, nccrp1, psca, DnaJC15, gsto1, and tk1) in susceptible (C57BL/6) versus resistant (BALB/c) mice was assessed by qRT-PCR. Of these molecules, expression of c1qb, DnaJC15, and tk1 was significantly higher (as defined by a P value of ≤0.05) in moribund C57BL/6 mice compared to resistant BALB/c mice, with fold increases of 17.3 ± 1.3, 3.7 ± 0.04, and 1.89 ± 0.005, respectively. Nccrp1, psca, and gsto1 had fold increases in expression of 7.1 ± 0.05, 5.7 ± 0.3, and 3.6 ± 0.5, respectively, which approached statistical significance (as defined by a P value of ≤0.1). These results demonstrate that circulatory host biomarkers identified by genome-wide gene expression profiling studies can be used as a diagnostic tool for ECM. Overall, our ability to detect the host biomarkers in blood that are closely associated with the clinical state of ECM offers the prospect of developing diagnostic tests for the prognosis and differential diagnosis of CM in hospital settings in countries where malaria is endemic. While some biologically relevant ECM biomarkers identified in our studies could be validated in clinical studies, extensive gene expression-profiling studies of blood samples from children during different clinical states of CM are needed to identify the biomarkers of human CM.
Supplementary Material
Acknowledgments
We thank Alvaro Godinez and Qin Su from NIAID, NIH, for expert help with microarray studies.
Editor: J. H. Adams
Footnotes
Published ahead of print on 13 December 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Balaji, S., L. M. Iyer, and L. Aravind. 2009. HPC2 and ubinuclein define a novel family of histone chaperones conserved throughout eukaryotes. Mol. Biosyst. 5:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beare, N. A., T. E. Taylor, S. P. Harding, S. Lewallen, and M. E. Molyneux. 2006. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am. J. Trop. Med. Hyg. 75:790-797. [PMC free article] [PubMed] [Google Scholar]
- 3.Berriz, G. F., J. E. Beaver, C. Cenik, M. Tasan, and F. P. Roth. 2009. Next generation software for functional trend analysis. Bioinformatics 25:3043-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolovan-Fritts, C. A., and S. A. Spector. 2008. Endothelial damage from cytomegalovirus-specific host immune response can be prevented by targeted disruption of fractalkine-CX3CR1 interaction. Blood 111:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. [DOI] [PubMed] [Google Scholar]
- 6.Casals-Pascual, C., et al. 2008. High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc. Natl. Acad. Sci. U. S. A. 105:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisada, S., et al. 2009. Zebrafish and mouse alpha2,3-sialyltransferases responsible for synthesizing GM4 ganglioside. J. Biol. Chem. 284:30534-30546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conroy, A. L., et al. 2009. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar. J. 8:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Souza, J. B., J. C. Hafalla, E. M. Riley, and K. N. Couper. 2010. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137:755-772. [DOI] [PubMed] [Google Scholar]
- 10.deWalick, S., et al. 2007. Cutting edge: conventional dendritic cells are the critical APC required for the induction of experimental cerebral malaria. J. Immunol. 178:6033-6037. [DOI] [PubMed] [Google Scholar]
- 11.Gauvreau, M. E., et al. 2009. Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic 10:1518-1527. [DOI] [PubMed] [Google Scholar]
- 12.Grau, G. E., et al. 2003. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 187:461-466. [DOI] [PubMed] [Google Scholar]
- 13.Grau, G. E., et al. 1986. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J. Immunol. 137:2348-2354. [PubMed] [Google Scholar]
- 14.Ha, J., et al. 2010. CXC chemokine ligand 2 induced by receptor activator of NF-kappa B ligand enhances osteoclastogenesis. J. Immunol. 184:4717-4724. [DOI] [PubMed] [Google Scholar]
- 15.Haggstrom Rudolfsson, S., A. Johansson, I. Franck Lissbrant, P. Wikstrom, and A. Bergh. 2003. Localized expression of angiopoietin 1 and 2 may explain unique characteristics of the rat testicular microvasculature. Biol. Reprod. 69:1231-1237. [DOI] [PubMed] [Google Scholar]
- 16.Han, M., et al. 2008. Novel blood-based, five-gene biomarker set for the detection of colorectal cancer. Clin. Cancer Res. 14:455-460. [DOI] [PubMed] [Google Scholar]
- 17.Ingley, E., P. A. Tilbrook, and S. P. Klinken. 2004. New insights into the regulation of erythroid cells. IUBMB Life 56:177-184. [DOI] [PubMed] [Google Scholar]
- 18.Ingvarsson, J., et al. 2008. Detection of pancreatic cancer using antibody microarray-based serum protein profiling. Proteomics 8:2211-2219. [DOI] [PubMed] [Google Scholar]
- 19.Kadri, Z., et al. 2009. Direct binding of pRb/E2F-2 to GATA-1 regulates maturation and terminal cell division during erythropoiesis. PLoS Biol. 7:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser, K., et al. 2006. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J. Infect. Dis. 193:987-995. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson, K. A. 1989. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58:309-350. [DOI] [PubMed] [Google Scholar]
- 22.Lackner, P., et al. 2008. Complement factors C1q, C3 and C5 in brain and serum of mice with cerebral malaria. Malar. J. 7:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamikanra, A. A., M. Theron, T. W. Kooij, and D. J. Roberts. 2009. Hemozoin (malarial pigment) directly promotes apoptosis of erythroid precursors. PLoS One 4:e8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepej, S. Z., et al. 2005. Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11. J. Neuroimmunol. 163:128-134. [DOI] [PubMed] [Google Scholar]
- 25.Looareesuwan, S., et al. 1983. Retinal hemorrhage, a common sign of prognostic significance in cerebral malaria. Am. J. Trop. Med. Hyg. 32:911-915. [DOI] [PubMed] [Google Scholar]
- 26.Lovegrove, F. E., et al. 2009. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4:e4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maglott, D., J. Ostell, K. D. Pruitt, and T. Tatusova. 2005. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 33:D54-D58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimento, E. J., et al. 2009. Gene expression profiling during early acute febrile stage of dengue infection can predict the disease outcome. PLoS One 4:e7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton, C. R., and S. Krishna. 1998. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol. Ther. 79:1-53. [DOI] [PubMed] [Google Scholar]
- 30.Oakley, M. S., et al. 2007. Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect. Immun. 75:2012-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakley, M. S., et al. 2009. Pathogenic roles of CD14, galectin-3, and OX40 during experimental cerebral malaria in mice. PLoS One 4:e6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakley, M. S., et al. 2008. Host biomarkers and biological pathways that are associated with the expression of experimental cerebral malaria in mice. Infect. Immun. 76:4518-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichiule, P., and J. C. LaManna. 2003. Expression of angiopoietin-1 and -2 in the rat brain during chronic hypoxia and de-adaptation. Adv. Exp. Med. Biol. 510:331-335. [DOI] [PubMed] [Google Scholar]
- 34.Randall, L. M., et al. 2008. Common strategies to prevent and modulate experimental cerebral malaria in mouse strains with different susceptibilities. Infect. Immun. 76:3312-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schotte, R., M. Nagasawa, K. Weijer, H. Spits, and B. Blom. 2004. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J. Exp. Med. 200:1503-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sexton, A. C., et al. 2004. Transcriptional profiling reveals suppressed erythropoiesis, up-regulated glycolysis, and interferon-associated responses in murine malaria. J. Infect. Dis. 189:1245-1256. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, T. E. 2009. Caring for children with cerebral malaria: insights gleaned from 20 years on a research ward in Malawi. Trans. R. Soc. Trop. Med. Hyg. 103(Suppl. 1):S6-S10. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, T. E., et al. 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 10:143-145. [DOI] [PubMed] [Google Scholar]
- 39.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Heyde, H. C., J. Nolan, V. Combes, I. Gramaglia, and G. E. Grau. 2006. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 22:503-508. [DOI] [PubMed] [Google Scholar]
- 41.Verschelde, C., et al. 2006. Overexpression of the antiapoptotic protein A1 promotes the survival of double positive thymocytes awaiting positive selection. Cell Death Differ. 13:1213-1221. [DOI] [PubMed] [Google Scholar]
- 42.Wassmer, S. C., et al. 2008. Platelet-induced clumping of Plasmodium falciparum-infected erythrocytes from Malawian patients with cerebral malaria—possible modulation in vivo by thrombocytopenia. J. Infect. Dis. 197:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheway, J., H. Herzog, and F. Mackay. 2007. NPY and receptors in immune and inflammatory diseases. Curr. Top. Med. Chem. 7:1743-1752. [DOI] [PubMed] [Google Scholar]
- 44.Wickramasinghe, S. N., R. E. Phillips, S. Looareesuwan, D. A. Warrell, and M. Hughes. 1987. The bone marrow in human cerebral malaria: parasite sequestration within sinusoids. Br. J. Haematol. 66:295-306. [DOI] [PubMed] [Google Scholar]
- 45.Wiese, L., C. Hempel, M. Penkowa, N. Kirkby, and J. A. Kurtzhals. 2008. Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar. J. 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.