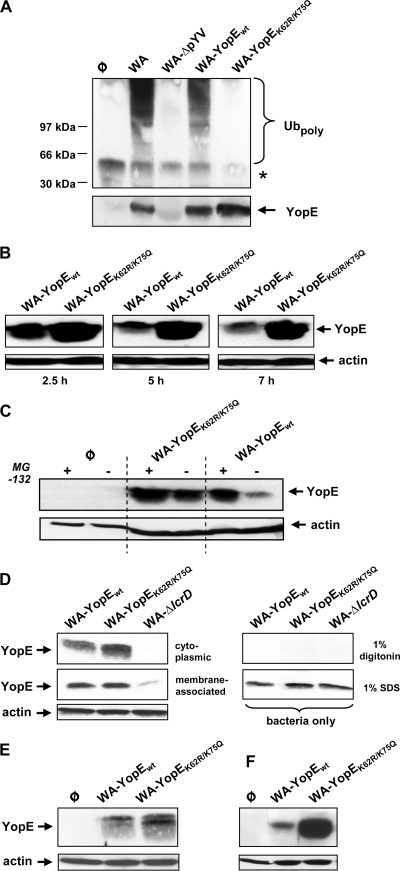
FIG. 3.
Mutagenesis of K62 and K75 prevents ubiquitination and proteasomal degradation of YopE from Y. enterocolitica serogroup O8. (A) Ubiquitination of YopE O8 protein species. HEK293 cells were left noninfected (φ) or infected with the wild-type Y. enterocolitica serogroup O8 strain WA, its virulence plasmid-cured derivative WA-ΔpYV, or the mutant strain recomplemented with either wild-type YopE (WA-YopEwt) or K62- and K75-mutagenized YopE (WA-YopEK62R/K75Q). Infections were performed in the presence of the proteasome inhibitor MG-132. Cellular extracts were prepared 75 min after onset of infection and immunoprecipitated using anti-YopE antibody. The immunoprecipitates were first immunoblotted with antiubiquitin for the detection of polyubiquitin-modified proteins (Ubpoly, upper panel). Subsequently, the membrane was stripped and reprobed with anti-YopE to control successful YopE precipitation (YopE, lower panel). The asterisk denotes the position of the H chain of the precipitating antibody. Molecular masses of standard marker proteins are indicated. (B) Time-dependent destabilization of wild-type YopE O8 in Y. enterocolitica-infected cells. HEK293 cells were infected with WA-YopEwt or WA-YopEK62R/K75Q. Ninety minutes later, yersiniae were killed by addition of gentamicin. At the denoted times after onset of infection, cellular lysates were prepared, cleared by centrifugation, and subjected to immunoblotting using anti-YopE antibody. (C) Stabilization of wild-type YopE O8 by proteasome inhibition. HEK293 cells were left uninfected (φ) or infected with the indicated Yersinia strains in the absence or presence of the proteasome inhibitor MG-132 as for panel B. At 3.5 h after onset of infection, cellular lysates were prepared, cleared by centrifugation, and analyzed on the cellular YopE level by immunoblotting. (D) Localization of YopE protein species to the cytoplasmic fraction of infected cells. HEK293 cells were infected with WA-YopEwt, WA-YopEK62R/K75Q, or the Yop secretion-defective mutant WA-ΔlcrD in the presence of MG-132. Ninety minutes later, cellular membranes were solubilized with 1% digitonin, and cleared from nonlysed cells, and bacteria by centrifugation. The cleared lysates were then subjected to ultracentrifugation. The YopE levels in the resulting cytoplasmic and membrane fractions were analyzed by immunoblotting (left panel). As a control for selective lysis of eukaryotic cells, bacteria were treated in the same manner with 1% digitonin in the absence of HEK293 cells or with 1% SDS to generate total bacterial cell lysates (right panel). (E) YopE protein species prepared from the cytoplasmic host cell fraction, as described for panel D, appear in higher resolution as a double band. (F) Differential stability of YopEwt and YopEK62R/K75Q in J774A.1 macrophages. J774A.1 cells were infected with WA-YopEwt or WA-YopEK62R/K75Q. At 3.5 h after onset of infection, cellular lysates were prepared and analyzed on the cellular YopE level by immunoblotting as for panel B. Equal loading of the gels with cellular, cytoplasmic lysates was controlled by stripping of the membranes and subsequent immunoblotting against actin.