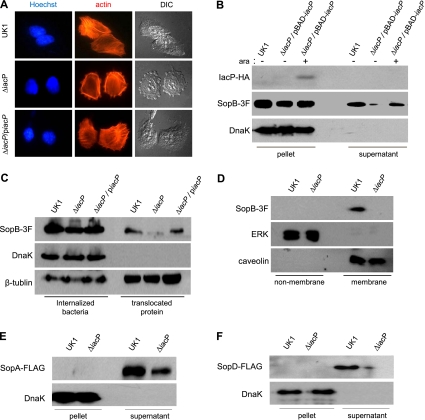
FIG. 4.
IacP promotes the T3SS-mediated secretion and translocation of SopB. (A) After bacterial infection for 15 min, filamentous actins were stained with rhodamine phalloidin. The nucleus and intracellular Salmonella serovar Typhimurium bacteria were stained with Hoechst 33342 dye (original magnification, ×1,260). (B) The secretion of SopB tagged with 3×FLAG (SopB-3F) in cultured supernatants was examined by Western blotting. l-Arabinose (0.2 mM) was added to induce the expression of IacP. Anti-DnaK antibody was used as a control for cytoplasmic proteins. (C) INT-407 cells infected with Salmonella serovar Typhimurium were treated with Triton X-100 (internalized bacteria) or SDS (translocated protein). Anti-β-tubulin antibody was used as a loading control. (D) To determine the subcellular localization of SopB, the membranes of infected INT-407 cells were isolated. Extracellular signal-regulated kinase (ERK) and caveolin were used as nonmembrane and membrane controls, respectively. (E and F) The culture supernatants of Salmonella serovar Typhimurium possessing SopA-FLAG (E) and SopD-FLAG (F) plasmids were immunoblotted with anti-FLAG antibody. Anti-DnaK antibody was used as a control for cytoplasmic proteins.