Fig. 6.
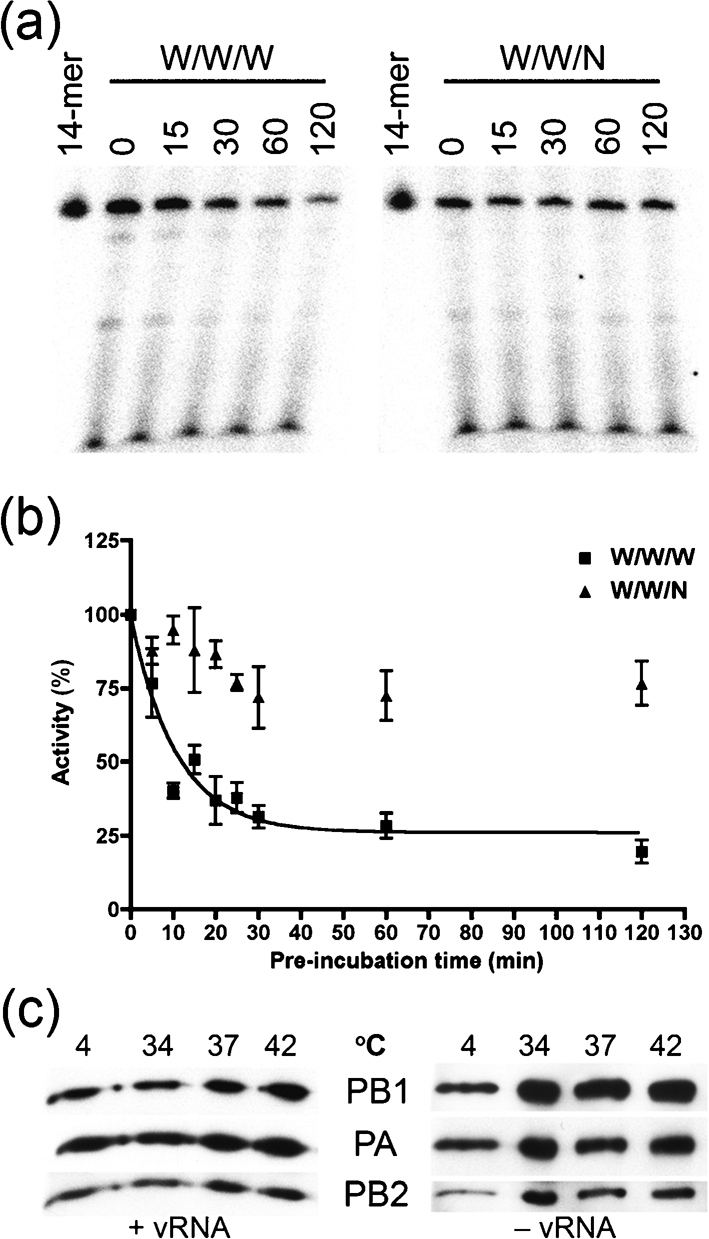
An avian PB2 subunit confers enhanced thermostability on the WSN 3P complex. Equivalent amounts of polymerase complex (as determined by polymerase activity at 30 °C) were pre-incubated at 30 °C in the absence of vRNA for different time periods. The complexes were then tested in an ApG-primed transcription assay (30 °C, 1 h). (a) A representative autoradiogram and (b) quantitative data from replicate experiments are shown. The data were normalized to the amount of fully extended product where no pre-incubation was performed (as quantified by Phosphorimager analysis). Mean transcriptional activities from at least four independent assays are shown. Bars, sem. A nonlinear curve for a single-phase exponential decay was fitted to the data using GraphPad Prism. The r2 value (goodness of fit) for the W/W/W curve was 0.81, and the half-life of the W/W/W complex was estimated at 7.3 min (with 95 % confidence intervals ranging from 5.3 to 11.8 min). The half-life of the W/W/N complex could not be determined, as it exceeded the 2 h of our analysis. (c) The purified WSN 3P complex was pre-incubated at the indicated temperatures in the presence (left panel) or absence of both 5′- and 3′-vRNA (right panel). The proteins were pulled-down with NiNTA Magnetic Agarose Beads and detected by immunoblotting with antibodies directed against PA (anti-His), PB1 and PB2.
