Abstract
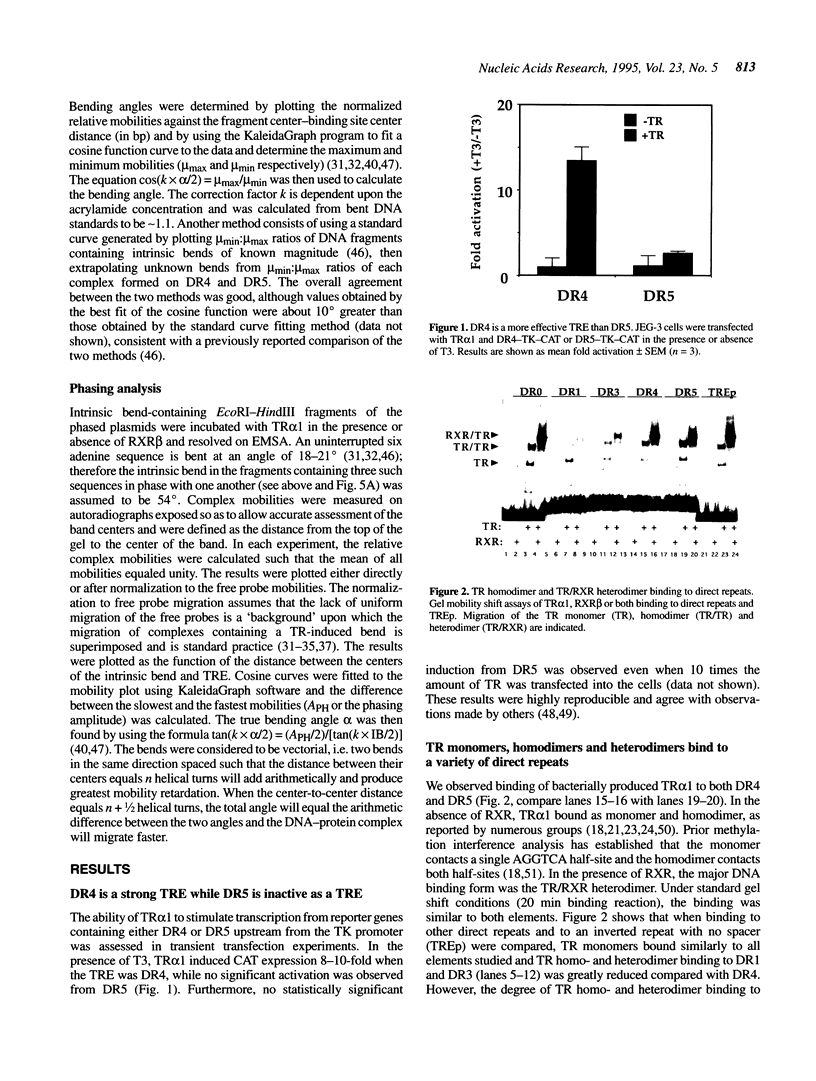
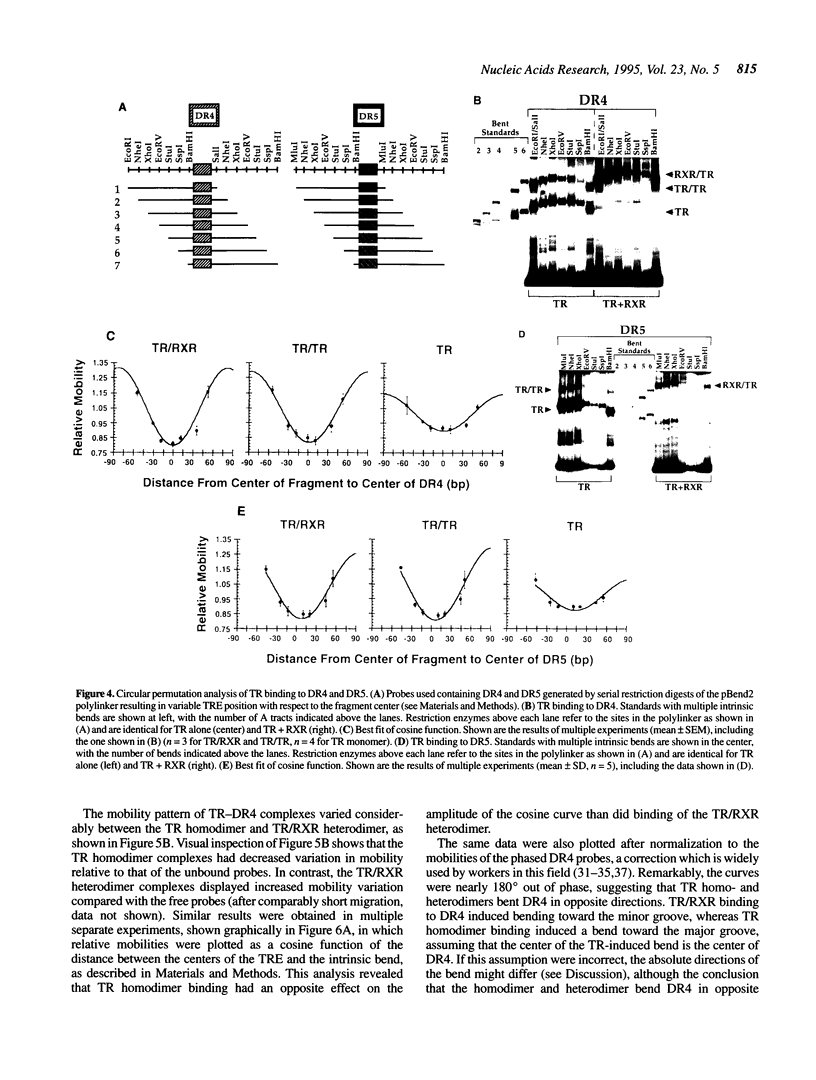
Transcriptional activation by thyroid hormone (T3) requires interactions between the T3 receptor (TR) and T3 response elements (TREs) composed of two copies of sequences related to AGGTCA. Direct repeats of this sequence are a functional TRE when spaced by 4 but not by 5 bp (DR4 versus DR5). TR bound as monomers, homodimers and heterodimers with retinoid X receptor (RXR) to both DR4 and DR5, with an approximately 10-fold greater affinity for DR4 due to reduced dissociation of the protein-DNA complex. We explored DNA bending as an additional variable which could influence the transcriptional outcome of the TR-TRE interaction. Circular permutation indicated a large distortion of the DNA following TR binding, but phasing analysis strongly suggested that this was due only in small part to DNA bending. Phasing analysis indicated that both TR/RXR and TR homodimer induced bends of approximately 10 degrees in DR4, but caused little bending of DR5. Moreover, the TR homo- and heterodimers bent DR4 in opposite directions. These results indicate that in addition to regulating the affinity and spacing requirement for DNA binding by TR, the TR dimer partner may also modulate transcription by influencing the direction of the bending induced by TR binding to DNA, although this effect may be subtle, due to the modest degree of bending.
Full text
PDF


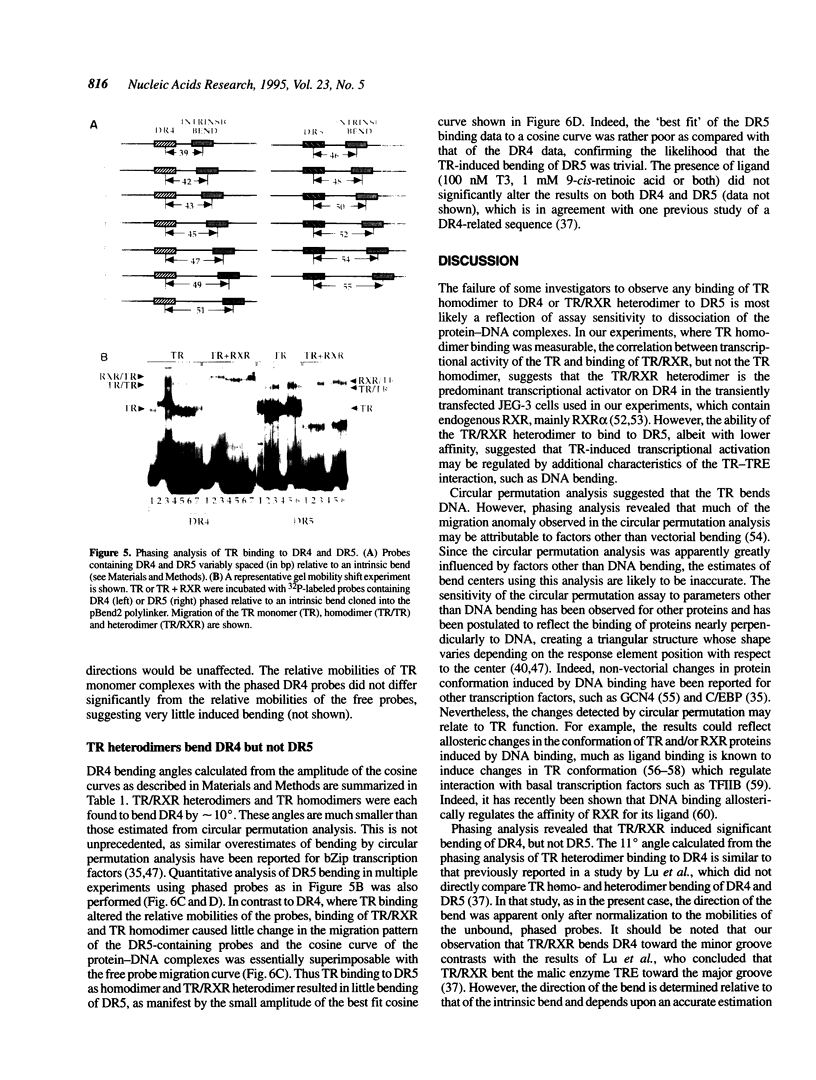
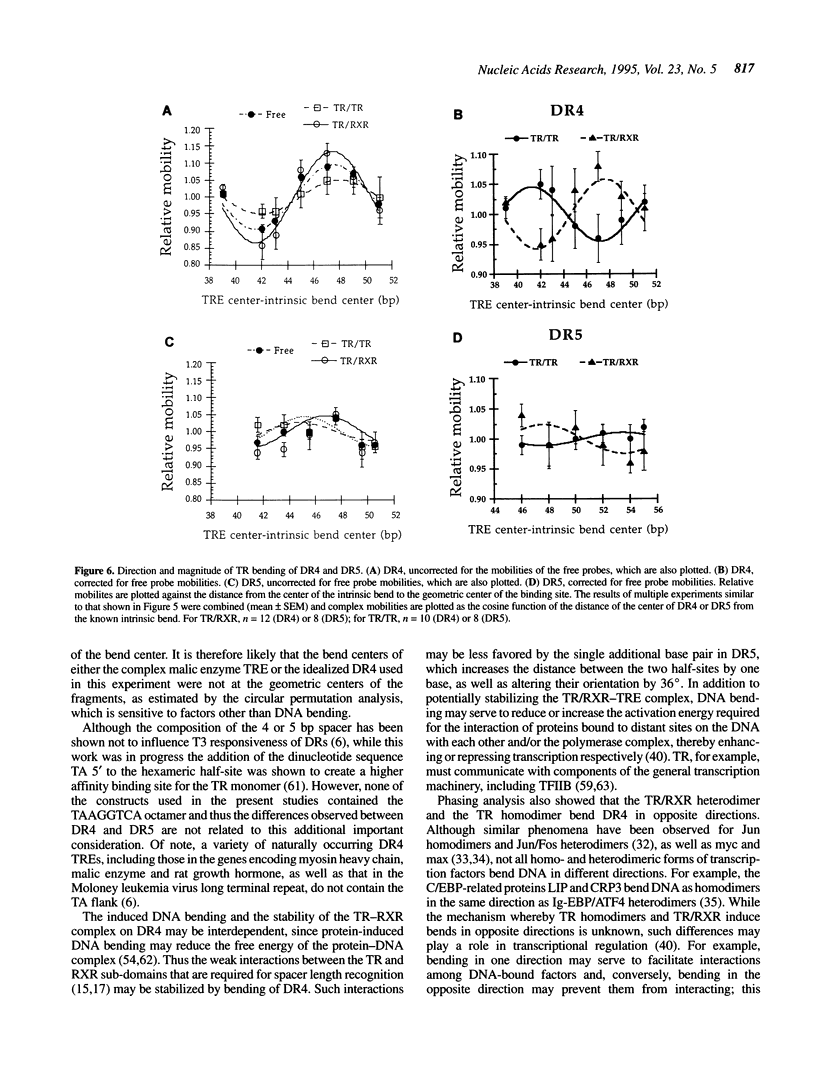

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M. L., Nordström K., Demczuk S., Harbers M., Vennström B. Thyroid hormone alters the DNA binding properties of chicken thyroid hormone receptors alpha and beta. Nucleic Acids Res. 1992 Sep 25;20(18):4803–4810. doi: 10.1093/nar/20.18.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au-Fliegner M., Helmer E., Casanova J., Raaka B. M., Samuels H. H. The conserved ninth C-terminal heptad in thyroid hormone and retinoic acid receptors mediates diverse responses by affecting heterodimer but not homodimer formation. Mol Cell Biol. 1993 Sep;13(9):5725–5737. doi: 10.1128/mcb.13.9.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitahl N., Calame K. The C/EBP family of proteins distorts DNA upon binding but does not introduce a large directed bend. J Biol Chem. 1994 Sep 23;269(38):23553–23562. [PubMed] [Google Scholar]
- Baniahmad A., Ha I., Reinberg D., Tsai S., Tsai M. J., O'Malley B. W. Interaction of human thyroid hormone receptor beta with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8832–8836. doi: 10.1073/pnas.90.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrodin T. J., Marks M. S., Ozato K., Linney E., Lazar M. A. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol Endocrinol. 1992 Sep;6(9):1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- Bhat M. K., Parkison C., McPhie P., Liang C. M., Cheng S. Y. Conformational changes of human beta 1 thyroid hormone receptor induced by binding of 3,3',5-triiodo-L-thyronine. Biochem Biophys Res Commun. 1993 Aug 31;195(1):385–392. doi: 10.1006/bbrc.1993.2055. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent G. A., Williams G. R., Harney J. W., Forman B. M., Samuels H. H., Moore D. D., Larsen P. R. Capacity for cooperative binding of thyroid hormone (T3) receptor dimers defines wild type T3 response elements. Mol Endocrinol. 1992 Apr;6(4):502–514. doi: 10.1210/mend.6.4.1584220. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Claverie-Martin F., Magasanik B. Positive and negative effects of DNA bending on activation of transcription from a distant site. J Mol Biol. 1992 Oct 20;227(4):996–1008. doi: 10.1016/0022-2836(92)90516-m. [DOI] [PubMed] [Google Scholar]
- Davis K. D., Berrodin T. J., Stelmach J. E., Winkler J. D., Lazar M. A. Endogenous retinoid X receptors can function as hormone receptors in pituitary cells. Mol Cell Biol. 1994 Nov;14(11):7105–7110. doi: 10.1128/mcb.14.11.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. E., Parent L. A., Sharp P. A. Myc/Max and other helix-loop-helix/leucine zipper proteins bend DNA toward the minor groove. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11779–11783. doi: 10.1073/pnas.89.24.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell J. D., Roy A. L., Roeder R. G. Unliganded thyroid hormone receptor inhibits formation of a functional preinitiation complex: implications for active repression. Genes Dev. 1993 Jul;7(7B):1400–1410. doi: 10.1101/gad.7.7b.1400. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Casanova J., Raaka B. M., Ghysdael J., Samuels H. H. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol Endocrinol. 1992 Mar;6(3):429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- Gartenberg M. R., Ampe C., Steitz T. A., Crothers D. M. Molecular characterization of the GCN4-DNA complex. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6034–6038. doi: 10.1073/pnas.87.16.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Hamada K., Gleason S. L., Levi B. Z., Hirschfeld S., Appella E., Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao E., Menke J. B., Smith A. M., Jones C., Geffner M. E., Hershman J. M., Wuerth J. P., Samuels H. H., Ways D. K., Usala S. J. Divergent dimerization properties of mutant beta 1 thyroid hormone receptors are associated with different dominant negative activities. Mol Endocrinol. 1994 Jul;8(7):841–851. doi: 10.1210/mend.8.7.7984146. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Rhee M., Chin W. W. Thyroid hormone receptor monomer, homodimer, and heterodimer (with retinoid-X receptor) contact different nucleotide sequences in thyroid hormone response elements. Endocrinology. 1994 Oct;135(4):1628–1638. doi: 10.1210/endo.135.4.7925126. [DOI] [PubMed] [Google Scholar]
- Kahn J. D., Yun E., Crothers D. M. Detection of localized DNA flexibility. Nature. 1994 Mar 10;368(6467):163–166. doi: 10.1038/368163a0. [DOI] [PubMed] [Google Scholar]
- Katz D., Berrodin T. J., Lazar M. A. The unique C-termini of the thyroid hormone receptor variant, c-erbA alpha 2, and thyroid hormone receptor alpha 1 mediate different DNA-binding and heterodimerization properties. Mol Endocrinol. 1992 May;6(5):805–814. doi: 10.1210/mend.6.5.1318505. [DOI] [PubMed] [Google Scholar]
- Katz D., Lazar M. A. Dominant negative activity of an endogenous thyroid hormone receptor variant (alpha 2) is due to competition for binding sites on target genes. J Biol Chem. 1993 Oct 5;268(28):20904–20910. [PubMed] [Google Scholar]
- Katz R. W., Koenig R. J. Nucleotide substitutions differentially affect direct repeat and palindromic thyroid hormone response elements. J Biol Chem. 1994 Apr 1;269(13):9500–9505. [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. DNA bending by Fos and Jun: the flexible hinge model. Science. 1991 Nov 22;254(5035):1210–1214. doi: 10.1126/science.1957173. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Selective DNA bending by a variety of bZIP proteins. Mol Cell Biol. 1993 Sep;13(9):5479–5489. doi: 10.1128/mcb.13.9.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zwieb C., Wu C., Adhya S. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene. 1989 Dec 21;85(1):15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- King I. N., de Soyza T., Catanzaro D. F., Lavin T. N. Thyroid hormone receptor-induced bending of specific DNA sequences is modified by an accessory factor. J Biol Chem. 1993 Jan 5;268(1):495–501. [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., DiRenzo J., Boehm M., Sugarman J., Gloss B., Rosenfeld M. G., Heyman R. A., Glass C. K. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994 Oct 6;371(6497):528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- Kurokawa R., Yu V. C., När A., Kyakumoto S., Han Z., Silverman S., Rosenfeld M. G., Glass C. K. Differential orientations of the DNA-binding domain and carboxy-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993 Jul;7(7B):1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Berrodin T. J., Harding H. P. Differential DNA binding by monomeric, homodimeric, and potentially heteromeric forms of the thyroid hormone receptor. Mol Cell Biol. 1991 Oct;11(10):5005–5015. doi: 10.1128/mcb.11.10.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Leidig F., Shepard A. R., Zhang W. G., Stelter A., Cattini P. A., Baxter J. D., Eberhardt N. L. Thyroid hormone responsiveness in human growth hormone-related genes. Possible correlation with receptor-induced DNA conformational changes. J Biol Chem. 1992 Jan 15;267(2):913–921. [PubMed] [Google Scholar]
- Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Ligand-dependent conformational changes in thyroid hormone and retinoic acid receptors are potentially enhanced by heterodimerization with retinoic X receptor. J Steroid Biochem Mol Biol. 1993 Dec;46(6):643–661. doi: 10.1016/0960-0760(93)90306-h. [DOI] [PubMed] [Google Scholar]
- Lu X. P., Eberhardt N. L., Pfahl M. DNA bending by retinoid X receptor-containing retinoid and thyroid hormone receptor complexes. Mol Cell Biol. 1993 Oct;13(10):6509–6519. doi: 10.1128/mcb.13.10.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Levi B. Z., Segars J. H., Driggers P. H., Hirschfeld S., Nagata T., Appella E., Ozato K. H-2RIIBP expressed from a baculovirus vector binds to multiple hormone response elements. Mol Endocrinol. 1992 Feb;6(2):219–230. doi: 10.1210/mend.6.2.1569965. [DOI] [PubMed] [Google Scholar]
- McAllister C. F., Achberger E. C. Rotational orientation of upstream curved DNA affects promoter function in Bacillus subtilis. J Biol Chem. 1989 Jun 25;264(18):10451–10456. [PubMed] [Google Scholar]
- Miyamoto T., Suzuki S., DeGroot L. J. High affinity and specificity of dimeric binding of thyroid hormone receptors to DNA and their ligand-dependent dissociation. Mol Endocrinol. 1993 Feb;7(2):224–231. doi: 10.1210/mend.7.2.8469235. [DOI] [PubMed] [Google Scholar]
- Nardulli A. M., Greene G. L., Shapiro D. J. Human estrogen receptor bound to an estrogen response element bends DNA. Mol Endocrinol. 1993 Mar;7(3):331–340. doi: 10.1210/mend.7.3.8483477. [DOI] [PubMed] [Google Scholar]
- Nelson C. C., Hendy S. C., Faris J. S., Romaniuk P. J. The effects of P-box substitutions in thyroid hormone receptor on DNA binding specificity. Mol Endocrinol. 1994 Jul;8(7):829–840. doi: 10.1210/mend.8.7.7984145. [DOI] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Paolella D. N., Palmer C. R., Schepartz A. DNA targets for certain bZIP proteins distinguished by an intrinsic bend. Science. 1994 May 20;264(5162):1130–1133. doi: 10.1126/science.8178171. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Rangarajan P. N., Umesono K., Evans R. M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993 Jul;7(7B):1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- Prost E., Moore D. D. CAT vectors for analysis of eukaryotic promoters and enhancers. Gene. 1986;45(1):107–111. doi: 10.1016/0378-1119(86)90138-1. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín J., Espinosa M. Protein-induced bending as a transcriptional switch. Science. 1993 May 7;260(5109):805–807. doi: 10.1126/science.8387228. [DOI] [PubMed] [Google Scholar]
- Ribeiro R. C., Kushner P. J., Apriletti J. W., West B. L., Baxter J. D. Thyroid hormone alters in vitro DNA binding of monomers and dimers of thyroid hormone receptors. Mol Endocrinol. 1992 Jul;6(7):1142–1152. doi: 10.1210/mend.6.7.1508227. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., O'Donnell A. L., Koenig R. J. Ligand-dependent synergy of thyroid hormone and retinoid X receptors. J Biol Chem. 1992 Nov 5;267(31):22010–22013. [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney J. H., Wu L., Summerfield A. E., Sanyal G., Forman B. M., Zhu J., Samuels H. H. Conformational changes in chicken thyroid hormone receptor alpha 1 induced by binding to ligand or to DNA. Biochemistry. 1993 Jan 12;32(1):2–6. doi: 10.1021/bi00052a001. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voz M. L., Peers B., Wiedig M. J., Jacquemin P., Belayew A., Martial J. A. Transcriptional regulation by triiodothyronine requires synergistic action of the thyroid receptor with another trans-acting factor. Mol Cell Biol. 1992 Sep;12(9):3991–3997. doi: 10.1128/mcb.12.9.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Dang C. V. Opposite orientations of DNA bending by c-Myc and Max. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7635–7639. doi: 10.1073/pnas.89.16.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. R., Harney J. W., Forman B. M., Samuels H. H., Brent G. A. Oligomeric binding of T3 receptor is required for maximal T3 response. J Biol Chem. 1991 Oct 15;266(29):19636–19644. [PubMed] [Google Scholar]
- Yen P. M., Darling D. S., Carter R. L., Forgione M., Umeda P. K., Chin W. W. Triiodothyronine (T3) decreases binding to DNA by T3-receptor homodimers but not receptor-auxiliary protein heterodimers. J Biol Chem. 1992 Feb 25;267(6):3565–3568. [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zechel C., Shen X. Q., Chambon P., Gronemeyer H. Dimerization interfaces formed between the DNA binding domains determine the cooperative binding of RXR/RAR and RXR/TR heterodimers to DR5 and DR4 elements. EMBO J. 1994 Mar 15;13(6):1414–1424. doi: 10.1002/j.1460-2075.1994.tb06395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Tran P. B., Pfahl M. DNA binding and dimerization determinants for thyroid hormone receptor alpha and its interaction with a nuclear protein. Mol Endocrinol. 1991 Dec;5(12):1909–1920. doi: 10.1210/mend-5-12-1909. [DOI] [PubMed] [Google Scholar]
- Zinkel S. S., Crothers D. M. Catabolite activator protein-induced DNA bending in transcription initiation. J Mol Biol. 1991 May 20;219(2):201–215. doi: 10.1016/0022-2836(91)90562-k. [DOI] [PubMed] [Google Scholar]