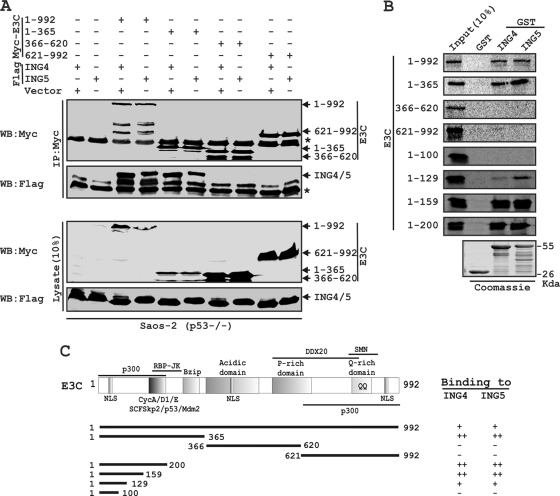
FIG. 2.
A critical N-terminal domain of EBNA3C interacts with both ING4 and ING5. (A) Saos-2 (p53−/−) cells were cotransfected with either flag-ING4 or -ING5 in the presence of different myc-EBNA3C constructs, as indicated. Cells were harvested, lysed, and immunoprecipitated with anti-myc antibody. Samples were resolved by a 7-to-15% gradient SDS-PAGE and detected by Western blotting (WB) for the indicated proteins by stripping and reprobing the same membrane. (B) The wild-type plasmid or plasmids of EBNA3C expressing different truncated mutant proteins were in vitro translated in the presence of [S35]Met. After preclearing of all S35-radiolabeled translated proteins with GST beads for 1 h at 4°C, samples were subjected to GST pulldown assay by incubating them with either a GST control, GST-ING4, or GST-ING5 as indicated. Reactions were resolved by appropriate SDS-PAGE, exposed to a phosphorimager plate, and scanned using the Storm 850 imaging system. Coomassie staining of a parallel SDS-PAGE gel resolved purified GST proteins, seen in the bottom panel. (C) The schematic illustrates different structural and interaction domains of EBNA3C and summarizes the results of studies of the binding between different domains of EBNA3C and ING4 and ING5. ++, strong binding; +, moderate binding; −, no binding. NLS, nuclear localization signal; Bzip, basic leucine zipper domain; SMN, survival of motor neurons. The asterisk indicates the immunoglobulin bands.