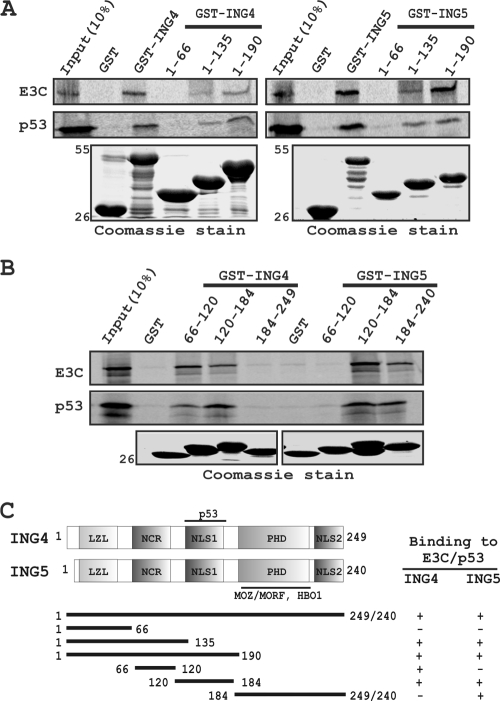
FIG. 5.
Both EBNA3C and p53 bind to the same region of either ING4 or -5. (A and B) Both S35-radiolabeled, in vitro-translated EBNA3C and p53 were subjected to binding reactions with either a GST control or different GST-fused, truncated proteins of both ING4 and ING5. Precipitated proteins were resolved by 10% SDS-PAGE, dried, exposed to a phosphorimager plate, and scanned. (A and B) Coomassie stains of SDS-PAGE-resolved purified GST-fused proteins are shown in the bottom panels. Numbers at the left indicate molecular masses in kilodaltons. (C) The schematic illustrates different structural and interaction domains of both ING4 and ING5 and summarizes the results of studies of the binding of ING4 and ING5 with p53 and EBNA3C. +, binding; −, no binding. LZL, leucine zipper-like motif; NCR, novel conserved region; NLS, nuclear localization signal; PHD, plant homeodomain.