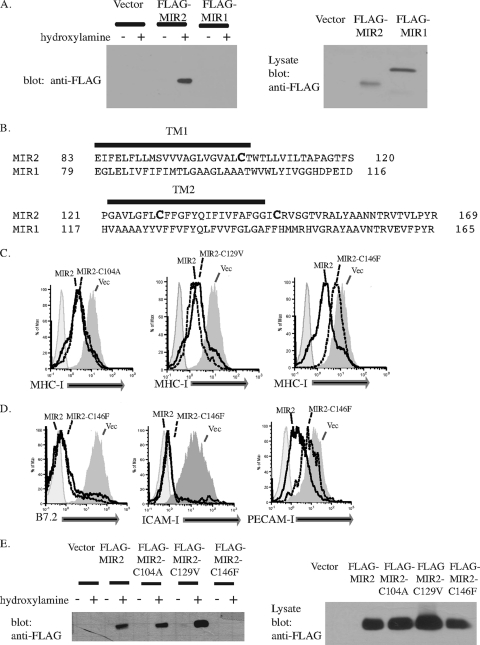
FIG. 3.
MIR2 is palmitoylated on cysteine 146. (A) Palmitoylation of MIR1 or MIR2 was detected by a biotin switch assay where protein palmitoylation is replaced with biotinylation. After transfection of HEK293T cells, total cell lysates were treated with N-ethylmaleimide to block free sulfhydral groups on unmodified cysteine residues. The lysate was treated (+) with hydroxylamine to remove palmitate or untreated (−). Free cysteines were then biotinylated with biotin-BMCC. Biotinylated proteins were then precipitated with NeutrAvidin beads (Pierce) and blotted for FLAG (left panel). Lysate samples from MIR-expressing cells were also probed with FLAG to verify MIR expression (right panel). Equal amounts of protein were loaded in each lane. (B) An alignment of MIR1 and MIR2 transmembrane domains. (C) MIR2 or MIR2 mutants fused to EGFP were individually transfected into HeLa cells. Twenty-four hours posttransfection, cells were stained with PE-conjugated antibody against MHC-I and analyzed by flow cytometry. Histograms were gated on GFP+ cells. (D) MIR2 or MIR2-C146F fused to EGFP was transiently transfected into HeLa cells stably transduced with B7.2 (left panel), FLAG-tagged ICAM-I (center panel), or FLAG-tagged PECAM-I (right panel). Cells were stained with mouse-raised primary antibody against B7.2 or against FLAG, stained with PE-conjugated secondary antibody against mouse IgG, and analyzed by flow cytometry. (E) The palmitoylation status of MIR2 mutants was determined using the biotin switch assay where protein palmitoylation is replaced with biotinylation. HEK 293T cells were transfected with individual MIR2 mutants, total cell lysates were subjected to the treatment described above for panel A, and the presence of biotinylated MIR2 was detected by probing Western blots for FLAG (left panel). Lysate samples from MIR2 or MIR2 mutant-expressing cells were also probed with FLAG to verify expression (right panel). Equal amounts of protein were loaded in each lane.