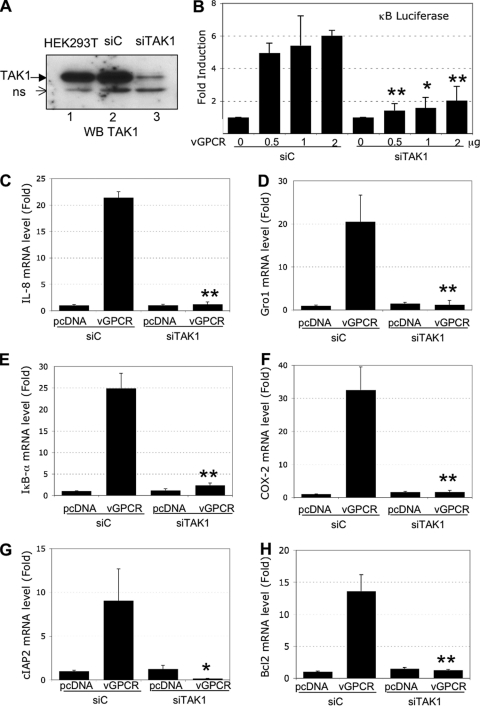
FIG. 4.
NF-κB activation induced by vGPCR is blocked by the expression of TAK1 siRNA. (A) TAK1 silencing. HEK293T cells were transduced with a lentivector expressing a control siRNA (siC) or an siRNA against TAK1 (siTAK1). One week later, the cells were lysed in RIPA lysis buffer, and 50 μg of protein was subjected to Western blot analysis using a TAK1 antibody. The detection of a nonspecific band (ns) was used as a loading control. (B) NF-κB luciferase activity induced by vGPCR is reduced when TAK1 is knocked down. HEK293T cells expressing siC or siTAK1 were transfected with pcDNA or increasing quantities of vGPCR expression plasmids, along with κB-luciferase and β-Gal reporter constructs (as described above). Thirty-six hours later, the luciferase and β-galactosidase activities were measured in triplicate using Steady-glo and β-glo Promega kits, respectively. The data represent the means and SD of fold induction (n = 3); *, P < 0.05; **, P < 0.01, comparing siTAK1 conditions with siC conditions with identical quantities of vGPCR-expressing plasmid. (C to H) HEK293T cells expressing siC or siTAK1 were transfected with or without 2 μg of vGPCR. Real-time PCR analysis measured the levels of message for the indicated genes. (C) IL-8. (D) Gro-1. (E) ΙκΒ-α. (F) COX-2. (G) cIAP2. (H) Bcl2. Each reaction was done in triplicate, and means and SD are shown. *, P < 0.05; **, P < 0.01, comparing vGPCR conditions in the siTAK1 cells with vGPCR conditions in the siC cells.