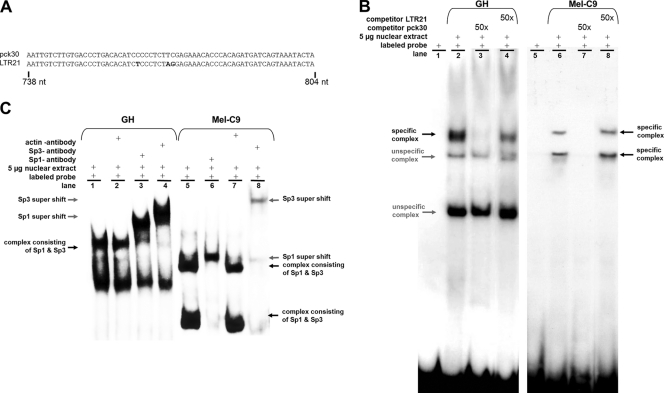
FIG. 7.
In vitro binding of Sp1 and Sp3 proteins to the GC box upstream of the TSS. (A) HERV-K LTR sequences generated by PCR as probes for EMSA. Nucleotide differences are depicted in bold. (B) Nuclear extracts of GH cells or Mel-C9 cells were incubated with the 32P-labeled LTRpck30 probe. Protein-probe complexes were separated by polyacrylamide gel electrophoresis and detected by autoradiography. A 50-fold molar excess of unlabeled LTRpck30 probe competed with the specific complexes (lane 3), but the unlabeled LTR21 probe did not (lane 4). (C) When anti-Sp1 or anti-Sp3 antibody was added to the EMSA reaction mixtures, the respective specific probe-protein complexes (black arrows) were shifted to complexes of higher molecular mass (supershift; gray arrows). Incubation with an anti-actin antibody did not induce a supershift.