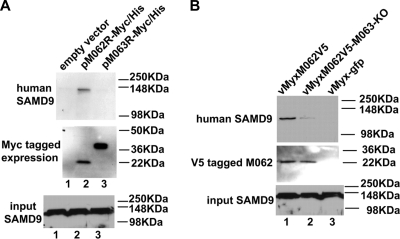
FIG. 7.
In human cells M062, but not M063, binds to endogenous SAMD9 protein, but the presence of M063 during MYXV infection enhances the interaction of M062 and SAMD9. (A) M062 binds to human SAMD9. HeLa cells were transfected with empty vector (pCDNA3.1-Myc/His) (lane 1), pM062R-Myc/His (lane 2), or pM063R-Myc/His (lane 3) for 48 h. Cells were lysed, and before a pull-down experiment using Ni-NTA beads was constructed, 1/50 volume of the cell lysate was saved for Western blotting to show the input SAMD9 (bottom panel). Proteins associated with the indicated transfected His-tagged proteins were pulled down, washed, and eluted from the beads. The eluted protein was separated by 10% SDS-PAGE and transferred to a PVDF membrane for immunoblotting. The membrane was first probed with anti-human SAMD9 antibody, stripped, and then probed with anti-myc antibody for myc-tagged protein expression. The SAMD9 protein was detected at a molecular mass of ∼148 kDa only in cells transfected with pM062R-Myc/His (top panel). The M063-myc tagged protein was detected at the observed molecular mass of 29.2 kDa, while the M062-myc tagged protein was detected at 23.7 kDa (middle panel). (B) The expression of M063 protein enhances the binding between M062 and SAMD9 in human cells during MYXV infection. U87 cells were infected with vMyxM062V5 (lane 1), vMyxM062V5-M063-KO (lane 2), or vMyx-gfp (lane 3) at an MOI of 1 for 48 h. Cells were harvested and, before co-IP using anti-V5 antibody was performed, a 1/50 volume of total cell lysate was saved for Western blotting to show SAMD9 input (bottom panel). Precipitated proteins were separated by 8% SDS-PAGE and transferred for an immunoblot procedure. The blot was first probed for SAMD9 expression (top panel). After stripping, it was then probed with anti-V5 antibody (middle panel).