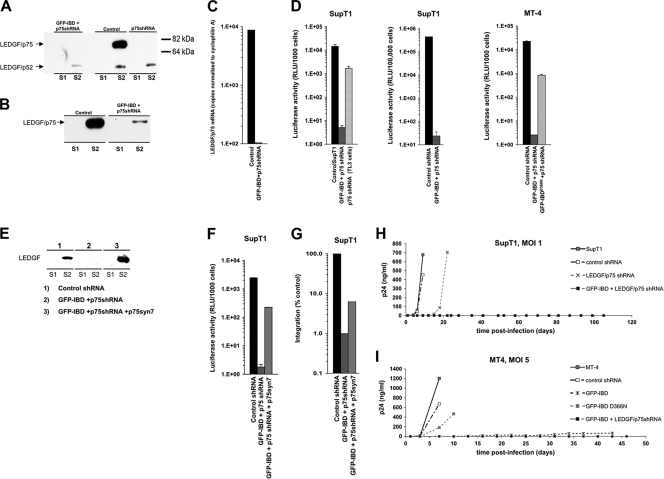
FIG. 5.
Combined dominant interference and knockdown. (A and B) Subcellular fractions of SupT1 (A) and MT4 cells (B) stably transduced with lentiviral vectors expressing LEDGF-targeted shRNA (p75shRNA) with or without GFP-IBD were analyzed for residual LEDGF expression using anti-LEDGF antibody. S1, Triton X-100-extractable, non-chromatin-bound fraction. S2, Triton X-100-resistant, chromatin-bound fraction releasable with salt and DNase treatment. See Fig. 3 in reference 32 for assay details and validation. (C) LEDGF mRNA levels in stable cell lines, assessed by real-time quantitative RT-PCR. The primers span the exons 14/15 junction and do not detect GFP-IBD. The results were normalized to copies of cyclophilin A mRNA. (D) SupT1 and MT4 cell lines stably expressing GFP-IBD and the LEDGF mRNA-targeting shRNA were challenged with HIV-1luc, and the luciferase activity was analyzed after 5 days. The fold inhibition values for the combined modalities in the three graphs from right to left are 2,860, 18,600, and 8,730, respectively. (E) SupT1 cells expressing both GFP-IBD and LEDGF shRNA were transduced with a retroviral vector encoding neoR and p75syn7, an LEDGF cDNA insensitive to the shRNA. Cells were selected in G418. Western blotting of subcellular fractions confirmed re-expression of LEDGF. (F and G) LEDGF re-expression partially restores HIV-1 infection in cells expressing GFP-IBD and LEDGF shRNA. A luciferase assay (F) and an Alu-PCR integration assay (G) were performed in SupT1 cell lines. (H and I) SupT1 (H) and MT4 (I) cells stably transduced with the indicated expression elements were challenged with HIV-1. Supernatant p24 antigen was determined at the various time points shown.