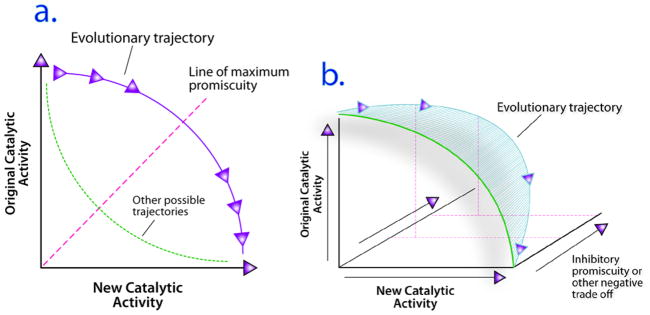
Figure 1.
A. Promiscuous Intermediate Model for Evolution of New Enzymatic Function. Enzymes with high catalytic specificity for one reaction (original catalytic activity) move along the indicated trajectory. At early stages they become promiscuous, gaining new catalytic activity without forfeiting the original activity. The dotted line indicates that other evolutionary trajectories are possible. B. Three-dimensional evolutionary trajectory relating catalytic function toward original and new substrates with susceptibility to inhibition. Susceptibility to inhibition could be a significant negative trade-off that increases the evolutionary distance from any intermediate to the desired evolutionary endpoint on the new activity axis. Each incremental increase in catalytic promiscuity has an associated cost of potential new inhibitory interaction with other ligands; the negative trade-off is revealed as an increase in the distance between the green two-dimensional curve and the blue trajectory as the catalytic promiscuity increases. The greater the increase in distance between the blue and green curves, the greater the negative trade-off. The detailed shape of the new trajectory is not known for any enzyme, but it ultimately restricts the possible evolutionary trajectories by defining the extent of negative trade-off.