Abstract
Ganciclovir (GCV), the therapy of choice for human cytomegalovirus (CMV) infections and foscarnet, a drug used to treat GCV-resistant CMV infections were approved more than twenty years ago. Although cidofovir and a prodrug of GCV have since been added to the armamentarium, a highly effective drug without significant toxicities has yet to be approved. Such a therapeutic agent is required for treatment of immunocompromised hosts and infants, which bear the greatest burden of disease. The modest antiviral activity of existing drugs is insufficient to completely suppress viral replication, which results in the selection of drug-resistant variants that remain pathogenic, continue to replicate, and contribute to disease. Sustained efforts, largely in the biotech industry and academia, have identified highly active lead compounds that have progressed into clinical studies with varying levels of success. A few of these compounds inhibit new molecular targets, remain effective against isolates that have developed resistance to existing therapies, and promise to augment existing therapies. Some of the more promising drugs will be discussed with an emphasis on those progressing to clinical studies. Their antiviral activity both in vitro and in vivo, spectrum of antiviral activity, and mechanism of action will be reviewed to provide an update on the progress of potential new therapies for CMV infections.
1. Background and Introduction
Significant advances have been made in the treatment of many of the infections caused by members of the herpesvirus family. The impressive efficacy of acyclovir and famciclovir against herpes simplex virus (HSV) and varicella-zoster virus infections provided the first examples of truly effective antiviral therapies and are used routinely to manage these infections (Dworkin et al., 2007; Leung and Sacks, 2000). Yet, the few approved therapies for other herpesviruses are wanting and new drugs are required. Human cytomegalovirus (CMV) shares many common characteristics with other members of the herpesvirus family, but significant biological differences including lengthy replication cycle, increased coding capacity, and comparatively high sequence diversity set it apart. The modest antiviral activity of currently approved therapies coupled with dose-limiting toxicities limits their effectiveness and often results in the development of resistance, particularly in immunocompromised hosts. New therapies are required that have improved efficacy as well as reduced toxicity to allow extended courses of therapy to suppress viral replication in the target population. The search for such therapies has identified several new inhibitors with superior antiviral activity but currently remain unproven in clinical studies. Candidate molecules in various stages of development will be discussed below and compared with existing therapies to provide perspective on their potential advantages.
2. Need for new therapies to treat CMV infections
Infection with CMV remains a significant problem in congenitally infected infants and immunocompromised individuals, including transplant recipients and those co-infected with human immunodeficiency virus (HIV) (Torres-Madriz and Boucher, 2008). This virus also infects up to 1% of all newborns and is the leading cause of brain damage and nonsyndromic sensorineural hearing loss in the United States (Morton and Nance, 2006; Stagno, 2001). Severe sequelae are associated with primary maternal infection and hearing loss occurs in almost half of infants with symptomatic congenital CMV infection (Fowler et al., 1992; James et al., 2009). Preexisting maternal immunity provides some measure of protection to the infant, although it is incomplete (Boppana et al., 1999; Ross et al., 2006). Detectable hearing loss also occurs in up to 7% of congenitally infected, but otherwise normal appearing, newborn infants (Nassetta et al., 2009; Rosenthal et al., 2009). While a six week course of ganciclovir (GCV) therapy to symptomatic infants has been reported to prevent further deterioration in hearing, it also appeared to induce the development of neutropenia during the course of treatment (Kimberlin et al., 2003). Costs associated with CMV hearing loss exceed $2 billion annually in the United States (Nassetta et al., 2009), thus better therapies to treat these infections may both improve health and reduce associated costs.
Infection with CMV is also a significant cause of morbidity and mortality in transplant recipients with severity of disease generally correlating with the degree of immunosuppression. Infection promotes events that lead to graft rejection following renal transplant, life-threatening pneumonitis in stem cell transplant recipients, and accelerated atherosclerosis following heart transplant (Griffiths, 2001; Potena and Valantine, 2007; Torres-Madriz and Boucher, 2008). The advent of highly effective HIV therapies has greatly reduced the incidence and severity of CMV infections, although maintenance is still required for some individuals and retinitis remains a cause of vision loss in this population (Kedhar and Jabs, 2007). The persistence of CMV infections in immunocompromised hosts generally requires long term therapy and the development of resistance to GCV is frequently observed in this population (Chou et al., 1997; Gilbert and Boivin, 2005; Scott et al., 2007). Resistance to this drug can arise with either preemptive or prophylactic therapy, with the highest incidence (5–10%) in seronegative transplant recipients receiving tissue from seropositive donors (Li et al., 2007; Limaye et al., 2000; Limaye et al., 2002; Lurain et al., 2002). Mutations that confer resistance typically map to the UL97 kinase, which catalyzes the initial phosphorylation of this drug, but can also be found in the DNA polymerase (Gilbert and Boivin, 2005). Many mutations that confer resistance to this drug also reduce susceptibility to second line therapies, although cross resistance to foscarnet (PFA) is somewhat less frequent (James et al., 2009).
3. Summary of approved therapies
Each of the three drugs approved and in use for the systemic therapy of CMV infections is effective (Jacobson, 1994), although moderate efficacy and dose limiting toxicities significantly limit their clinical utility (Andrei et al., 2008; Biron, 2006b; Griffiths, 2001). All of the compounds are related in that they target the viral DNA polymerase and inhibit viral DNA synthesis. An antisense phosphorothioate oligonucleotide complementary to the IE2 mRNA encoding IE2, formivirsen, has also been approved for the therapy of CMV retinitis in patients with HIV infection that are unable to receive other approved therapies for this condition (de Smet et al., 1999). Although the intravitreous injection of this compound has been reported to be effective (2002), it is used infrequently and will not be discussed further.
3.1 Ganciclovir and valganciclovir
GCV therapy has been shown to provide some clinical benefit in immunocompromised hosts (Jacobson, 1994), yet resistance to this drug occurs rather frequently in this population and appears to be related to low levels of viral replication that occur notwithstanding sustained GCV therapy (Emery and Griffiths, 2000; Griffiths, 2001). A prodrug of GCV, valganciclovir (VGCV), has been shown to have ten-fold greater oral bioavailability than the nucleoside (Jung and Dorr, 1999), provides adequate drug exposure (Perrottet et al., 2009), and has been shown to be effective against CMV infections in the clinic (Kimberlin et al., 2008; Snoeck et al., 2002).
Although the CMV DNA polymerase is the molecular target of the drug, it must first be phosphorylated to the active triphosphate metabolite. The initial phosphorylation step is specifically catalyzed by the UL97 kinase (Sullivan et al., 1992), and two subsequent phosphorylation steps are performed by host cellular kinases (Faulds and Heel, 1990). Competitive inhibition of the viral DNA polymerase by GCV triphosphate results in the termination of DNA elongation (Biron et al., 1985). Since GCV is a substrate for the UL97 kinase and GCV triphosphate is a substrate for DNA polymerase, mutations that confer resistance to GCV can occur in either or both open reading frames.
3.2 Cidofovir
Therapy with cidofovir (CDV) has also been shown to be effective in the treatment of CMV infections in individuals infected with HIV, however, its nephrotoxicity and lack of oral bioavailability has generally limited its use (Jacobson, 1997; Safrin et al., 1997). This drug is an acyclic deoxycytidine monophosphate analog with antiviral activity against a very broad range of DNA viruses including the herpesviruses, adenoviruses, polyomaviruses, and orthopoxviruses (De Clercq and Holy, 2005; De Clercq et al., 1986; Snoeck and De Clercq, 2002; Snoeck et al., 1988). No initial phosphorylation step by UL97 kinase is required; the conversion to the diphosphoryl metabolite, which inhibits the DNA polymerase, is catalyzed by cellular enzymes (Cihlar and Chen, 1996).
3.3 Foscarnet
Foscarnet (PFA) can also be effective in the treatment of CMV infections in those co-infected with HIV (Walmsley et al., 1988). This pyrophosphate analog reversibly inhibits DNA polymerase in many herpesviruses by binding to and blocking the viral polymerase’s pyrophosphate binding site (Wagstaff and Bryson, 1994). This noncompetitive inhibition interferes with pyrophosphate cleavage from incoming deoxynucleoside triphosphates and results in the inhibition of viral DNA synthesis (Crumpacker, 1992). Because PFA acts directly on viral DNA polymerase, it does not require any activation by either viral or host phosphorylative enzymes. This compound is also useful for the treatment GCV-resistant infections, although its associated renal toxicity limits its usefulness (Torres-Madriz and Boucher, 2008).
3.4 Resistance to approved therapies
GCV remains the therapy of choice for CMV infections, but resistance to this molecule can be problematic in the target population of immunocompromised hosts (Gilbert et al., 2002; Griffiths, 2001). The persistent nature of CMV infections in this population requires prolonged therapy to suppress viral replication and the modest efficacy of GCV drives the development of resistance that arises when low levels of viral replication persist in the presence of the drug (Drew, 2000; Emery and Griffiths, 2000; Limaye et al., 2000). The development of resistance has proven to be a significant concern in solid organ transplant recipients (Ahmed et al., 2004; Zamora, 2004). Although the emergence of GCV-resistant viruses in human stem cell transplant recipients has been reported (Marfori et al., 2007), it appears to be less common in this population than in solid organ transplant recipients (Bhorade et al., 2002; Gilbert et al., 2001; Nichols et al., 2001). Most infections that are resistant to GCV remain susceptible to PFA since cross-resistance between these drugs is rare and is typically mediated by mutations in different genes. More than 90% of GCV-resistant isolates contain one or more mutations in UL97 kinase (Erice, 1999), which catalyzes the phosphorylation of the drug (Sullivan et al., 1992). Since the enzymatic activity of UL97 kinase is critical for viral replication (Gill et al., 2009; Prichard et al., 1999), mutations associated with resistance are typically point mutations or small deletions in conserved regions of the kinase that impair GCV phosphorylation, yet do not substantially impact kinase activity (Gilbert and Boivin, 2005). Most mutations occur in conserved regions with codons 460, 594, and 595 accounting for around 70% of GCV-resistant CMV strains (Boivin et al., 2001; Chou et al., 2002). By contrast, mutations in the DNA polymerase gene, UL54, can confer resistance to GCV, CDV, or PFA and the distribution of polymorphisms associated with resistance is much more diffuse. Mutations have been described in conserved regions II, IV, V, IV and δ-region C (Chou et al., 2000; Cihlar et al., 1998; Gilbert et al., 2002; Scott et al., 2007).
4. Specific inhibitors of CMV replication
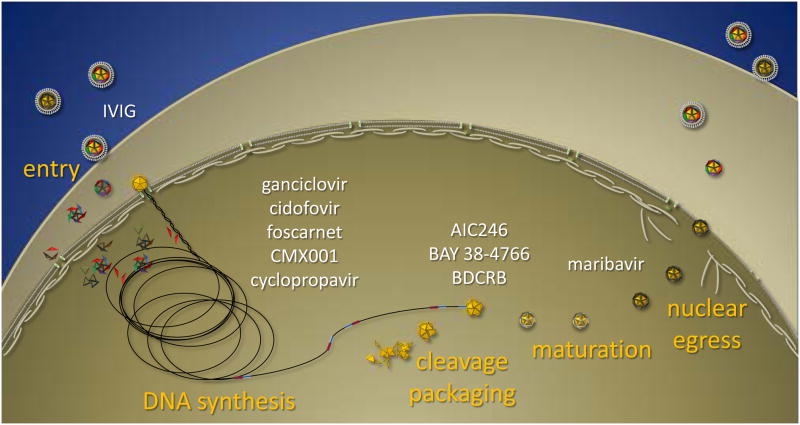
The replication cycle of CMV is complex and offers a wealth of potential targets. The search for novel inhibitors has not only taken advantage of conventional targets, such as the DNA polymerase, but has resulted in the discovery of new molecular targets and greatly improved our understanding of the biology of this virus. Novel inhibitors of CMV infection will be reviewed with respect to their efficacy, spectrum of antiviral activity, and their molecular targets insofar as they have been described. Although many inhibitors of cellular targets have been identified that impact viral replication, this review will focus primarily on inhibitors that specifically target viral gene products with an emphasis on those with increased potential to become new therapies, i.e. are highly effective in the treatment of CMV infections in experimental model infections or in phase 2 clinical studies. A broader review of all compounds that inhibit CMV replication, including those that target cellular processes, was published recently by De Clercq and coworkers (Andrei et al., 2008; Andrei et al., 2009). Here, compounds will be presented in order of the stage of viral replication they block to highlight those with novel molecular targets. A summary of the virus life cycle and selected compounds that disrupt each stage is depicted in Figure 1.
Figure 1.
Summary of compounds that inhibit various stages of CMV replication. Infection is initiated by virions that bind to receptors at the cell surface and can be prevented through the inactivation of virions by purified immunoglobulins (IVIG) or other attachment inhibitors. Several nucleoside inhibitors are capable of inhibiting the subsequent synthesis of viral DNA, including the approved therapies. The coordinated steps of genome cleavage/packaging are inhibited by three separate classes of inhibitors and prevent the stable encapsidation of nascent viral DNA. Inhibitors of the viral UL97 kinase, such as maribavir, impact both early and late events in viral replication and inhibit the egress of mature capsids into the cytoplasm.
4.1 Attachment inhibitors
CMV replication commences with binding events at the cell surface that are mediated by viral glycoproteins and results in the penetration of the nucleocapsid into the cytoplasm (Britt and Mach, 1996). Preparations of intravenous immune globulin (IVIG) have been shown to neutralize CMV and theoretically could impact disease by interfering with the binding of the virus to host cells, however, its efficacy in transplant recipients appears to be nominal and has been difficult to document (Sokos et al., 2002). More specific therapies have been developed against glycoprotein B (gB), an abundant membrane glycoprotein that is essential for viral infectivity and provides initial interactions with the cell via heparin sulfate (Compton et al., 1993). Inhibitors of membrane fusion have been developed using β-amino acid oligomers that mimic the heptad repeats of gB (English et al., 2006). These inhibitors are specific for CMV, inhibit fusion, and are thought to interact with the heptad repeat segment of gB or gH and block either homo- or hetero-protein-protein associations required to induce membrane fusion. A small molecule fusion inhibitor, CFI02, has also been identified that blocks membrane fusion and can inhibit the cell-cell spread of the virus (Jones et al., 2004). Further development of any attachment inhibitors will likely require clinical trials since animal models of CMV infection are currently inadequate to evaluate such an approach.
4.2 Inhibitors of DNA synthesis
The synthesis of viral DNA represents the next stage of viral replication targeted by specific inhibitors (Fig. 2). Indeed, a wealth of active compounds has been described that inhibit the DNA polymerase including the approved therapies GCV, CDV, and PFA, as well as non nucleoside inhibitors (Wathen, 2002). Another highly effective class of new compounds is the acyclic nucleoside phosphonates, some of which have antiviral activity against CMV (De Clercq and Holy, 2005). The efficacy of these analogs can also be increased tremendously through the formation of alkoxyalkyl ester prodrugs, which improve pharmacokinetics as well as oral bioavailability (Hostetler, 2009). Molecules undergoing active development will be summarized.
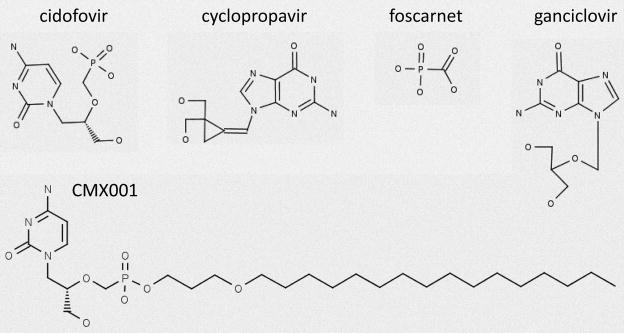
Figure 2.
Selected inhibitors of viral DNA synthesis.
4.2.1 CDV, CMX001, and other nucleoside phosphonate analogs
Cidofovir has greater activity against CMV in vitro and in vivo than any of the other licensed drugs for the treatment of CMV infection (Hitchcock et al., 1996; Kern, 1991; Kern et al., 2001; Snoeck et al., 1988), and is approved for the treatment of CMV retinitis. Although CDV exhibits potent antiviral activity against CMV, it lacks oral bioavailability, has dose limiting toxicity, and often results in severe nephrotoxicity (Lalezari et al., 1997; Safrin et al., 1997). The synthesis of alkylglycerol phosphate or alkylpropyl phosphate esters of ACV, penciclovir, or GCV were reported previously to improve antiviral activity when administered orally in animal models of HSV, experimental models of CMV, or hepatitis infections (Hostetler, 2009; Hostetler et al., 1997; Hostetler et al., 2001). A similar approach was used to improve the oral bioavailability of CDV and a series of ether lipid ester analogs of CDV or cyclic CDV (cCDV) were prepared and evaluated for activity against herpesviruses and poxviruses (Beadle et al., 2002; Keith et al., 2004).
We have reported previously that this series of ether lipid ester prodrugs of CDV, particularly hexadecyloxypropyl CDV (HDP-CDV, now known as CMX001) and octadecyloxyethyl CDV (ODE-CDV) analogs exhibited greatly enhanced in vitro efficacy against all the human herpesviruses including CMV (Williams-Aziz et al., 2005). It has also been reported that some of these analogs including CMX001 were 1000-fold more potent in vitro than CDV against CMV and MCMV (Wan et al., 2005). The analogs retained their activity against drug resistant isolates that have mutations in the UL97 and UL54 genes and remain active against ganciclovir-resistant isolates of CMV. We have also reported that CMX001 and ODE-CDV had markedly enhanced activity in vitro against vaccinia and cowpox viruses (Keith et al., 2004), and were shown to have activity when administered orally in pharmokinetic (Ciesla et al., 2003), and efficacy studies in mice (Kern et al., 2004b; Quenelle et al., 2004). This drug is broadly active against other DNA viruses including BK virus and adenovirus (Hartline et al., 2005; Randhawa et al., 2006).
Since there are no animal models that closely simulate human CMV disease, the surrogate viruses, murine CMV(MCMV), rat CMV and guinea pig CMV have been used for preclinical evaluation of potential therapies for CMV infections (Bravo et al., 2006; Glasgow et al., 1982; Kern, 1991; Kern et al., 2004b; Li et al., 1990; Stals et al., 1991). We have used the MCMV model extensively for evaluation of new compounds and have reported previously that CDV and the alkoxyalkyl analogs had greater activity than GCV (Kern, 1991; Kern, 1997; Kern, 1999). In addition, CDV and the phosphonate analogs also exhibited good therapeutic activity against HCMV infections in SCID mice implanted with human retinal or thymus/liver tissue (Bidanset et al., 2004a; Bidanset et al., 2004b; Kern et al., 2001).
In experiments reported previously, the efficacy of CMX001 and ODE-CDV given orally was compared with that of CDV given parenterally for their ability to alter mortality and virus replication in target organs of mice infected with MCMV (Kern et al., 2004b). Since CDV has been reported to have a long intracellular half-life (Cundy et al., 1996), it is still efficacious when administered to mice 1–3 times weekly, rather than once or twice daily (Kern, 1991; Quenelle et al., 2003). It was further determined that these ether lipid esters, such as CMX001, were also effective in reducing mortality of mice when administered either once daily, twice weekly, or as a single dose (Kern et al., 2004b).
Further studies in vivo to evaluate CMX001 confirmed that the compound was effective when administered orally against MCMV (Kern et al., 2004b), CMV (Bidanset et al., 2004a), orthopoxviruses (Parker et al., 2008; Quenelle et al., 2004), and adenoviruses (Toth et al., 2008). Additionally, combinations of CMX001 and ST-246, an inhibitor of late stage poxvirus replication, were also shown to synergistically reduce mortality in animals infected with cowpox virus which confirmed the in vitro combination results (Quenelle et al., 2007b).
The antiviral activity of related alkoxyalkyl esters of HPMPA also translated into in vivo efficacy against CMV and MCMV (Quenelle et al., 2008), as well as the orthopoxviruses (Quenelle et al., 2007a). Related alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine also exhibited good activity against CMV and the orthopoxviruses (Beadle et al., 2006). Similarly, alkoxyalkyl esters of phosphonopropoxymethyl-guanine and phosphonopropoxymethyl-diaminopurine also potently inhibited CMV, HSV and the orthopoxviruses (Ruiz et al., 2006; Ruiz et al., 2007). A similar strategy using 5-phosphono-pent-2-en-1-yl nucleosides and their alkoxyalkyl phosphonoesters also improved the efficacy of the compounds against CMV and other DNA viruses (Choo et al., 2007). In fact, this approach appears to improve the efficacy of purine and pyrimidine nucleoside phosphonates generally and also resulted in greatly improved efficacy against HIV (Valiaeva et al., 2006), as well as the herpesviruses (Prichard et al., 2008). Improvements in efficacy were sufficiently large that it effectively expanded the spectrum of activity of the compounds and suggests that this approach might be used to develop broad spectrum antiviral drugs with activity against HIV as well as the herpesviruses. The increased in vitro efficacy afforded by the esterification with alkoxyalkyl groups also resulted in the reevaluation of (S)-3-hydroxy-2-(phosphonomethoxy)propyl analogs of guanine, adenine, cytosine, diaminopurine and thymidine (Valiaeva et al., 2009). This analysis showed that the ODE esters of all of the analogs exhibited good to excellent antiviral activity against vaccinia virus and the herpesviruses, whereas the unesterified compounds were poorly active or inactive.
The in vitro and animal model data summarized above taken together with results from pharmacokinetic, metabolic, and toxicologic studies were used to select CMX001 as a candidate for clinical evaluation (Painter and Hostetler, 2004; Painter et al., 2008), and CMX001 has successfully completed Phase 1 clinical trials. This compound proved to be well tolerated and confirmed earlier studies in mice that showed significantly reduced accumulation of the compound in the kidney, therefore avoiding the nephrotoxicity that is observed frequently with the parent compound, CDV (Ciesla et al., 2003; Painter et al., 2008; Quenelle et al., 2010). CMX001 is currently being evaluated in Phase 2 clinical studies for treatment of BK virus and CMV infections (ClinicalTrials.gov identifier: NCT00793598 and NCT00942305), and for single cases of adenovirus and poxvirus infections.
4.2.2 Cyclopropavir and other methylenecyclopropane analogs
The methylenecyclopropanes represent a relatively new series of molecules modeled after allene analogs, which have been shown to exhibit antiviral activity (Zemlicka, 1993). The first generation of these analogs was essentially bioisosteric analogs of acyclovir where the C-O-C moiety was replaced by the methylenecyclopropane moiety. In a similar fashion, the second generation of methylenecyclopropane analogs is related more closely to ganciclovir (Zhou et al., 2004a). Both Z- and E-isomeric analogs were generated for each series of compounds. We have reported previously that Z isomers of the first generation of methylenecyclopropane analogs are potent agents against some members of the herpesvirus family (Chen et al., 2003; Kushner et al., 2003; Qiu et al., 2000; Qiu et al., 1998a; Qiu et al., 1999; Qiu et al., 1998b; Rybak et al., 2000; Rybak et al., 1999). These analogs had strong antiviral activity against CMV, MCMV, rat CMV, rhesus monkey CMV, guinea pig CMV (Rybak et al., 2000), human herpesvirus 6 (HHV-6) (Kushner et al., 2003; Rybak et al., 2000; Zemlicka, 2002), and human herpesvirus 8 (HHV-8) (Kushner et al., 2003). Some of these compounds significantly reduced mortality and virus replication in animal models of human and murine CMV (Kern et al., 2004a; Rybak et al., 1999). More recently, a second generation of methylenecyclopropane analogs, the 2, 2-bis-hydroxymethyl derivatives were synthesized (Zhou et al., 2004a). One member of this new series of compounds, ZSM-I-62, which has been given the name cyclopropavir (CPV), has been reported to be very effective in preventing mortality of mice infected with MCMV and in reducing replication of virus in visceral organs from mice infected with MCMV and in human fetal tissue implanted into SCID mice and infected with CMV (Kern et al., 2004a). This compound, CPV, was evaluated for activity against all the human herpesviruses and we reported that it was active in vitro against CMV, HHV-6A, HHV-6B and HHV-8 (Kern et al., 2005). In CMV infection, this compound was reported to require the UL97 kinase for its antiviral activity, but retained activity against most GCV-resistant isolates and clinical isolates. More recent work showed that the UL97 kinase is stereoselective and phosphorylates CPV to its (+)-monophosphate (Gentry et al., 2010).
Further studies indicated that phosphonate analogs of cyclopropavir also had antiviral activity against CMV and retained activity against GCV-resistant isolates with mutations in the UL97 kinase as would be expected for these monophosphate analogs (Mhaske et al., 2009). Valine esters of CPV also appeared to offer improved pharmacokinetic parameters over the parent compound (Wu et al., 2009). Another class of halogenated methylenecyclopropane analogs was identified including the (Z)- and (E)-[2-Fluoro-2-(hydroxymethyl)cyclopropylidene]methyl-purines and -pyrimidines, which exhibited activity against CMV, HSV, VZV, and EBV (Zhou et al., 2009; Zhou et al., 2004b; Zhou and Zemlicka, 2007; Zhou et al., 2007). The lead molecule for the methylenecyclopropane series, CPV, is highly active against CMV in vitro and in vivo and will soon be evaluated in phase 1 clinical studies.
4.3 Cleavage/Packaging of viral DNA
Considerable quantities of viral DNA accumulate during CMV replication, yet little is packaged into mature virions. Replicative intermediates appear to form very large, potentially branched concatemers that require further processing to resolve unit length genomes suitable for packaging in capsids (Courcelle and Mocarski, 2001; Pari, 2008). Terminase subunits encoded by UL89 and UL56 together with the virion portal protein encoded by UL104 form a complex that directs the cleavage/packaging of viral genomes and has been reviewed recently (Bogner, 2002). This essential process presents novel targets for antiviral chemotherapy and at least three classes of inhibitors have been identified (Fig. 3).
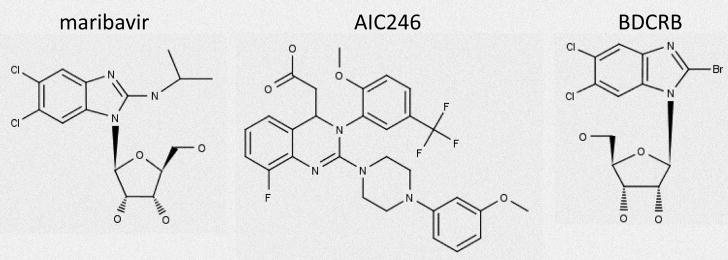
Figure 3.
Selected inhibitors of later steps in viral replication.
4.3.1 Benzimidazole Ribosides
Halogenated benzimidazole analogs were the first molecules identified that blocked the cleavage/packaging of viral genomes and were shown to eliminate the formation of monomer viral genomes in infected cells (Underwood et al., 1998). The prototype compound, 1H-β-D-ribofuranosyl-2-bromo-5,6-dichlorobenzimidazole (BDCRB) is an excellent inhibitor of CMV replication both in vitro (Townsend et al., 1995), and in vivo (Kern et al., 2004c). This series of compounds has been recently reviewed (Drach et al., 2006). In infected cells treated with BDCRB, DNA packaging is initiated, yet cleavage signals are not recognized (McVoy and Nixon, 2005). Although this molecule is a nucleoside analog, it does not require phosphorylation for antiviral activity (Krosky et al., 2002), and targets components of the viral terminase (Krosky et al., 1998). The rapid degradation of this molecule by 8-oxoguanine DNA glycosylase and N-methylpurine DNA glycosylase precluded further development (Lorenzi et al., 2006), but a more stable analog, GW275175X, was synthesized that appears to have similar mechanism of action (Underwood et al., 2004) and retains good activity against CMV (Kern et al., 2004c; Williams et al., 2003). Of these compounds, GW275175X is the best candidate for further development; however, no clinical studies are currently in progress.
4.3.2 BAY 38-4766
A second series of compounds were identified that also inhibit the packaging of CMV DNA including the lead compound, BAY 38-4766 (Reefschlaeger et al., 2001). This report indicated that its spectrum of antiviral activity was broader than that of BDCRB, and exhibited good antiviral activity in mice infected with MCMV. The molecule also possessed good pharmacokinetic parameters and was well tolerated in animals (McSharry et al., 2001a). Despite structural differences with BDCRB, mutations that confer resistance to its antiviral activity also map to both UL56 and UL89 and confirm that the terminase complex is the ultimate molecular target (Buerger et al., 2001).
4.3.3. AIC246
A third class of inhibitors with excellent antiviral activity has been recently described with AIC246 as the lead compound (Lischka et al., 2010). Although components of the terminase have not been formally ascribed as the targets of this molecule, it appears to inhibit a very late stage in viral replication and likely inhibits cleavage/packaging. This molecule was inactive against MCMV, but retained efficacy against several clinical isolates of CMV, including GCV-resistant isolates. It also inhibited the replication of CMV in human cells using the gelfoam implant model of CMV infection in mice (Lischka et al., 2010). Currently, the drug is in phase 2 clinical trials and has been reported to be well tolerated.
4.4 Inhibitors of UL97 protein kinase
The UL97 kinase phosphorylates a host of viral and cellular proteins and is important for the replication of the virus. Pleiotropic effects that occur in the absence of its enzymatic activity make it difficult to unambiguously identify a stage of viral replication affected by its inhibitors (Prichard, 2009). Since their most profound effects occur late in infection, they are included in a separate category with other inhibitors of late events.
Critical functions performed by the UL97 kinase make it an excellent target for the development of antiviral drugs (Prichard, 2009). Although this kinase is not essential for virus replication, the absence of its activity severely compromises the ability of the virus to replicate and results in virus titers that are reduced by at least two orders of magnitude (Prichard et al., 1999). Several inhibitors of its activity have been identified and represent a new class of antiviral drugs (Biron et al., 2002; Herget and Marschall, 2006; Mercorelli et al., 2008). These include indolocarbazoles (Marschall et al., 2001; Marschall et al., 2002; Zimmermann et al., 2000), quinazolines (Herget et al., 2004), as well as benzimidazole analogs (Biron et al., 2002). With the exception of maribavir, none of these compounds have progressed to clinical studies.
4.4.1 Maribavir
Maribavir (MBV) is a benzimidazole L riboside and is the only highly specific inhibitor of the UL97 kinase, and this literature has been reviewed recently (Biron, 2006a). It exhibits favorable pharmacokinetic properties, is well tolerated, and holds promise as a new drug for the treatment of CMV infections (Koszalka et al., 2002; Lalezari et al., 2002; Ma et al., 2006). Inhibition of UL97 kinase activity by MBV was established early in the development of the drug and its complex mechanism of action corresponds to the UL97 null phenotype (Prichard, 2009; Prichard et al., 1999). Studies with the drug have contributed significantly to our understanding of UL97 function and have been reviewed recently (Chou, 2008). Drug resistant mutants can be selected in the laboratory, and arise more frequently in strains of the virus that contain mutations in the exonuclease domain II of the DNA polymerase (Chou and Marousek, 2008). Resulting mutations are distinct from those of GCV-resistant mutants and some lie outside the conserved kinase domains (Chou, 2008; Chou et al., 2002; Chou et al., 2007). Consistent with these observations, the drug remains active against GCV-resistant strains and should be useful in the treatment of drug resistant infections (Biron et al., 2002; McSharry et al., 2001b; Williams et al., 2003). However, the inhibition of UL97 kinase activity by MBV may interfere with the activation of GCV if administered concomitantly (Evers et al., 2002), and it has been reported to occur in cell culture (Chou and Marousek, 2006). Clinical studies need to be designed with this issue in mind, but strategies to minimize this effect clearly exist. Interestingly, most resistant strains isolated in the laboratory do not have mutations in UL97, but rather map to the UL27 gene (Chou et al., 2004; Komazin et al., 2004). The resistance conferred by mutations within UL27 is modest compared to those in the kinase, but they appear to occur much more frequently. The protein product of this gene, pUL27, has no reported function, but its deletion results in a modest half log reduction in viral replication in vitro with no apparent effect on replication in vivo (Prichard et al., 2006). It is unclear how pUL27 impacts the activity of MBV, but it is possible that it modulates pUL97 activity by some mechanism and our understanding of these effects will elucidate an important new aspect of the UL97 phenotype. In a phase 3 clinical study drug-treated patients did not meet the clinical end-point objectives and the future of this drug is uncertain.
5.0 Conclusions
Existing drugs for the therapy of CMV infections have proven to be relatively safe and effective, yet their associated toxicities and modest efficacy after long term therapy leaves these infections underserved. These shortcomings are particularly apparent in immunocompromised hosts, besieged by insidious infections that require extended therapy. The incomplete suppression of viral replication afforded by existing agents leads to the selection of drug-resistant variants. Development of additional therapies with improved efficacy and reduced potential for toxicity is clearly required.
Progress towards better therapies for CMV infections has been rather slow and reflects difficulties in finding effective agents that can be administered for prolonged periods without any untoward side effects. The development of HAART therapies for HIV infection has reduced the numbers of patients requiring therapy for CMV retinitis and resulted in the cessation of many programs focused on the development of new therapies. However, numbers of immunosuppressed individuals are increasing as organ transplantation has become more common and a few new compounds are progressing into clinical trials. It is critical that these efforts continue.
Combination therapy will likely be required to treat infections in immunocompromised hosts to improve the current standard of care. The development of new molecules that target viral proteins other than the DNA polymerase will be particularly valuable in such therapeutic regimens. Obvious candidate combinations would likely include polymerase inhibitors, as well as other compounds with distinct molecular targets to minimize the development of resistance. Given that extended therapies are required to suppress viral replication in immunocompromised hosts, combinations of agents will likely be required to keep residual viral replication to a minimum and prevent the selection of resistant isolates. Ideal combination therapies would exhibit broad spectrum antiviral activity against not only CMV, but all the human herpesviruses that plague individuals with a compromised immune system and could improve significantly the health and long term outcome of this target population.
Acknowledgments
This work was supported by Public Health Service contract N01-AI-30049 from the NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed J, Velarde C, Ramos M, Ismail K, Serpa J, Ortigosa-Goggins M, Parasuraman R, Venkat KK. Outcome of low-dose ganciclovir for cytomegalovirus disease prophylaxis in renal-transplant recipients. Transplantation. 2004;78(11):1689–1692. doi: 10.1097/01.tp.0000141364.85454.37. [DOI] [PubMed] [Google Scholar]
- Andrei G, De Clercq E, Snoeck R. Novel inhibitors of human CMV. Curr Opin Investig Drugs. 2008;9(2):132–145. [PubMed] [Google Scholar]
- Andrei G, De Clercq E, Snoeck R. Drug targets in cytomegalovirus infection. Infect Disord Drug Targets. 2009;9(2):201–222. doi: 10.2174/187152609787847758. [DOI] [PubMed] [Google Scholar]
- Beadle JR, Hartline C, Aldern KA, Rodriguez N, Harden E, Kern ER, Hostetler KY. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob Agents Chemother. 2002;46(8):2381–2386. doi: 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle JR, Wan WB, Ciesla SL, Keith KA, Hartline C, Kern ER, Hostetler KY. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine against cytomegalovirus and orthopoxviruses. J Med Chem. 2006;49(6):2010–2015. doi: 10.1021/jm050473m. [DOI] [PubMed] [Google Scholar]
- Bhorade SM, Lurain NS, Jordan A, Leischner J, Villanueva J, Durazo R, Creech S, Vigneswaran WT, Garrity ER. Emergence of ganciclovir-resistant cytomegalovirus in lung transplant recipients. J Heart Lung Transplant. 2002;21(12):1274–1282. doi: 10.1016/s1053-2498(02)00463-1. [DOI] [PubMed] [Google Scholar]
- Bidanset DJ, Beadle JR, Wan WB, Hostetler KY, Kern ER. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J Infect Dis. 2004a;190(3):499–503. doi: 10.1086/421912. [DOI] [PubMed] [Google Scholar]
- Bidanset DJ, Rybak RJ, Hartline CB, Kern ER. Efficacy of ganciclovir and cidofovir against human cytomegalovirus replication in SCID mice implanted with human retinal tissue. Antiviral Res. 2004b;63(1):61–64. doi: 10.1016/j.antiviral.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Biron K. Maribavir: a promising new antiherpes therapeutic agent. In: Bogner E, Holzenburg A, editors. New concepts of antiviral therapy. Springer; Dordrecht, the Netherlands: 2006a. p. xviii.p. 538. [Google Scholar]
- Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006b;71(2–3):154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA, 3rd, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46(8):2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron KK, Stanat SC, Sorrell JB, Fyfe JA, Keller PM, Lambe CU, Nelson DJ. Metabolic activation of the nucleoside analog 9-[(2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12(2):115–127. doi: 10.1002/rmv.344. [DOI] [PubMed] [Google Scholar]
- Boivin G, Gilbert C, Gaudreau A, Greenfield I, Sudlow R, Roberts NA. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J Infect Dis. 2001;184(12):1598–1602. doi: 10.1086/324672. [DOI] [PubMed] [Google Scholar]
- Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics. 1999;104(1 Pt 1):55–60. doi: 10.1542/peds.104.1.55. [DOI] [PubMed] [Google Scholar]
- Bravo FJ, Cardin RD, Bernstein DI. Effect of maternal treatment with cyclic HPMPC in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2006;193(4):591–597. doi: 10.1086/499603. [DOI] [PubMed] [Google Scholar]
- Britt WJ, Mach M. Human cytomegalovirus glycoproteins. Intervirology. 1996;39(5–6):401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- Buerger I, Reefschlaeger J, Bender W, Eckenberg P, Popp A, Weber O, Graeper S, Klenk HD, Ruebsamen-Waigmann H, Hallenberger S. A novel nonnucleoside inhibitor specifically targets cytomegalovirus DNA maturation via the UL89 and UL56 gene products. J Virol. 2001;75(19):9077–9086. doi: 10.1128/JVI.75.19.9077-9086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kern ER, Drach JC, Gullen E, Cheng YC, Zemlicka J. Structure-activity relationships of (S,Z)-2-aminopurine methylenecyclopropane analogues of nucleosides. Variation of purine-6 substituents and activity against herpesviruses and hepatitis B virus. J Med Chem. 2003;46(8):1531–1537. doi: 10.1021/jm0205245. [DOI] [PubMed] [Google Scholar]
- Choo H, Beadle JR, Kern ER, Prichard MN, Keith KA, Hartline CB, Trahan J, Aldern KA, Korba BE, Hostetler KY. Antiviral activities of novel 5-phosphono-pent-2-en-1-yl nucleosides and their alkoxyalkyl phosphonoesters. Antimicrob Agents Chemother. 2007;51(2):611–615. doi: 10.1128/AAC.00444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev Med Virol. 2008;18(4):233–246. doi: 10.1002/rmv.574. [DOI] [PubMed] [Google Scholar]
- Chou S, Marousek G, Guentzel S, Follansbee SE, Poscher ME, Lalezari JP, Miner RC, Drew WL. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J Infect Dis. 1997;176(3):786–789. doi: 10.1086/517302. [DOI] [PubMed] [Google Scholar]
- Chou S, Marousek GI. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob Agents Chemother. 2006;50(10):3470–3472. doi: 10.1128/AAC.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Marousek GI. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J Virol. 2008;82(1):246–253. doi: 10.1128/JVI.01787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Marousek GI, Senters AE, Davis MG, Biron KK. Mutations in the human cytomegalovirus UL27 gene that confer resistance to maribavir. J Virol. 2004;78(13):7124–7130. doi: 10.1128/JVI.78.13.7124-7130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Miner RC, Drew WL. A deletion mutation in region V of the cytomegalovirus DNA polymerase sequence confers multidrug resistance. J Infect Dis. 2000;182(6):1765–1768. doi: 10.1086/317618. [DOI] [PubMed] [Google Scholar]
- Chou S, Waldemer RH, Senters AE, Michels KS, Kemble GW, Miner RC, Drew WL. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J Infect Dis. 2002;185(2):162–169. doi: 10.1086/338362. [DOI] [PubMed] [Google Scholar]
- Chou S, Wechel LC, Marousek GI. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J Infect Dis. 2007;196(1):91–94. doi: 10.1086/518514. [DOI] [PubMed] [Google Scholar]
- Ciesla SL, Trahan J, Wan WB, Beadle JR, Aldern KA, Painter GR, Hostetler KY. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59(3):163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Chen MS. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol Pharmacol. 1996;50(6):1502–1510. [PubMed] [Google Scholar]
- Cihlar T, Fuller MD, Cherrington JM. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J Virol. 1998;72(7):5927–5936. doi: 10.1128/jvi.72.7.5927-5936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193(2):834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- Courcelle CT, Mocarski ES. Cytomegaloviruses and their replication. In: Fields BN, Knipe DM, Howley PM, Griffin DE, editors. Fields virology. 4. Lippincott Williams & Wilkins; Philadelphia: 2001. p. xix.p. 3087. [Google Scholar]
- Crumpacker CS. Mechanism of action of foscarnet against viral polymerases. Am J Med. 1992;92(2A):3S–7S. doi: 10.1016/0002-9343(92)90329-a. [DOI] [PubMed] [Google Scholar]
- Cundy KC, Bidgood AM, Lynch G, Shaw JP, Griffin L, Lee WA. Pharmacokinetics, bioavailability, metabolism, and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab Dispos. 1996;24(7):745–752. [PubMed] [Google Scholar]
- De Clercq E, Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat Rev Drug Discov. 2005;4(11):928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Holy A, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323(6087):464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- de Smet MD, Meenken CJ, van den Horn GJ. Fomivirsen - a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul Immunol Inflamm. 1999;7(3–4):189–198. doi: 10.1076/ocii.7.3.189.4007. [DOI] [PubMed] [Google Scholar]
- Drach J, Townsend L, Bogner E. Benzimidazoled-ribonucleosides as antiviral agents that target HCMV terminase. In: Bogner E, Holzenburg A, editors. New concepts of antiviral therapy. Springer; Dordrecht, the Netherlands: 2006. p. xviii.p. 538. [Google Scholar]
- Drew WL. Ganciclovir resistance: a matter of time and titre. Lancet. 2000;356(9230):609–610. doi: 10.1016/S0140-6736(00)02597-6. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, Nurmikko TJ, Oaklander AL, Oxman MN, Pavan-Langston D, Petersen KL, Rowbotham MC, Schmader KE, Stacey BR, Tyring SK, van Wijck AJ, Wallace MS, Wassilew SW, Whitley RJ. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- Emery VC, Griffiths PD. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc Natl Acad Sci U S A. 2000;97(14):8039–8044. doi: 10.1073/pnas.140123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English EP, Chumanov RS, Gellman SH, Compton T. Rational development of beta-peptide inhibitors of human cytomegalovirus entry. J Biol Chem. 2006;281(5):2661–2667. doi: 10.1074/jbc.M508485200. [DOI] [PubMed] [Google Scholar]
- Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev. 1999;12(2):286–297. doi: 10.1128/cmr.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DL, Komazin G, Shin D, Hwang DD, Townsend LB, Drach JC. Interactions among antiviral drugs acting late in the replication cycle of human cytomegalovirus. Antiviral Res. 2002;56(1):61–72. doi: 10.1016/s0166-3542(02)00094-3. [DOI] [PubMed] [Google Scholar]
- Faulds D, Heel RC. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1990;39(4):597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- Gentry BG, Kamil JP, Coen DM, Zemlicka J, Drach JC. Stereoselective Phosphorylation of Cyclopropavir by pUL97 and Competitive Inhibition by Maribavir. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.00468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C, Bestman-Smith J, Boivin G. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Updat. 2002;5(2):88–114. doi: 10.1016/s1368-7646(02)00021-3. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Boivin G. Human cytomegalovirus resistance to antiviral drugs. Antimicrob Agents Chemother. 2005;49(3):873–883. doi: 10.1128/AAC.49.3.873-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C, Roy J, Belanger R, Delage R, Beliveau C, Demers C, Boivin G. Lack of emergence of cytomegalovirus UL97 mutations conferring ganciclovir (GCV) resistance following preemptive GCV therapy in allogeneic stem cell transplant recipients. Antimicrob Agents Chemother. 2001;45(12):3669–3671. doi: 10.1128/AAC.45.12.3669-3671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RB, Frederick SL, Hartline CB, Chou S, Prichard MN. Conserved retinoblastoma protein-binding motif in human cytomegalovirus UL97 kinase minimally impacts viral replication but affects susceptibility to maribavir. Virol J. 2009;6:9. doi: 10.1186/1743-422X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow LA, Richards JT, Kern ER. Effect of acyclovir treatment on acute and chronic murine cytomegalovirus infection. Am J Med. 1982;73(1A):132–137. doi: 10.1016/0002-9343(82)90078-x. [DOI] [PubMed] [Google Scholar]
- Griffiths PD. Cytomegalovirus therapy: current constraints and future opportunities. Curr Opin Infect Dis. 2001;14(6):765–768. doi: 10.1097/00001432-200112000-00016. [DOI] [PubMed] [Google Scholar]
- Hartline CB, Gustin KM, Wan WB, Ciesla SL, Beadle JR, Hostetler KY, Kern ER. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis. 2005;191(3):396–399. doi: 10.1086/426831. [DOI] [PubMed] [Google Scholar]
- Herget T, Freitag M, Morbitzer M, Kupfer R, Stamminger T, Marschall M. Novel chemical class of pUL97 protein kinase-specific inhibitors with strong anticytomegaloviral activity. Antimicrob Agents Chemother. 2004;48(11):4154–4162. doi: 10.1128/AAC.48.11.4154-4162.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget T, Marschall M. Recent developments in anti-herpesviral therapy based on protein kinase inhibitors. In: Bogner E, Holzenburg A, editors. New concepts of antiviral therapy. Springer; Dordrecht, the Netherlands: 2006. p. xviii.p. 538. [Google Scholar]
- Hitchcock MJ, Jaffe HS, Martin JC, Stagg RJ. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir Chem Chemother. 1996;7:115–127. [Google Scholar]
- Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82(2):A84–98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler KY, Beadle JR, Kini GD, Gardner MF, Wright KN, Wu TH, Korba BA. Enhanced oral absorption and antiviral activity of 1-O-octadecyl-sn-glycero-3-phospho-acyclovir and related compounds in hepatitis B virus infection, in vitro. Biochem Pharmacol. 1997;53(12):1815–1822. doi: 10.1016/s0006-2952(97)82446-x. [DOI] [PubMed] [Google Scholar]
- Hostetler KY, Rybak RJ, Beadle JR, Gardner MF, Aldern KA, Wright KN, Kern ER. In vitro and in vivo activity of 1-O-hexadecylpropanediol-3-phospho-ganciclovir and 1-O-hexadecylpropanediol-3-phospho-penciclovir in cytomegalovirus and herpes simplex virus infections. Antivir Chem Chemother. 2001;12(1):61–70. doi: 10.1177/095632020101200104. [DOI] [PubMed] [Google Scholar]
- Jacobson MA. Current management of cytomegalovirus disease in patients with AIDS. AIDS Res Hum Retroviruses. 1994;10(8):917–923. doi: 10.1089/aid.1994.10.917. [DOI] [PubMed] [Google Scholar]
- Jacobson MA. Treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337(2):105–114. doi: 10.1056/NEJM199707103370207. [DOI] [PubMed] [Google Scholar]
- James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83(3):207–213. doi: 10.1016/j.antiviral.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TR, Lee SW, Johann SV, Razinkov V, Visalli RJ, Feld B, Bloom JD, O’Connell J. Specific inhibition of human cytomegalovirus glycoprotein B-mediated fusion by a novel thiourea small molecule. J Virol. 2004;78(3):1289–1300. doi: 10.1128/JVI.78.3.1289-1300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39(8):800–804. doi: 10.1177/00912709922008452. [DOI] [PubMed] [Google Scholar]
- Kedhar SR, Jabs DA. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Herpes. 2007;14(3):66–71. [PubMed] [Google Scholar]
- Keith KA, Wan WB, Ciesla SL, Beadle JR, Hostetler KY, Kern ER. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication in vitro. Antimicrob Agents Chemother. 2004;48(5):1869–1871. doi: 10.1128/AAC.48.5.1869-1871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER. Value of animal models to evaluate agents with potential activity against human cytomegalovirus. Transplant Proc. 1991;23(3 Suppl 3):152–155. discussion 155. [PubMed] [Google Scholar]
- Kern ER. Preclinical evaluation of antiviral agents. In: Galasso GJ, Whitley RJ, Merigan TC, editors. Antiviral agents and human viral diseases. 4. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 79–111. [Google Scholar]
- Kern ER. Animal Models for cytomegalovirus infections: murine CMV. In: Zak O, Sande M, Carbon C, Kaninsky R, O’Reilly T, Kern ER, editors. Handbook on animal models for antimicrobial therapy. Academic Press; London, England: 1999. pp. 927–934. [Google Scholar]
- Kern ER, Bidanset DJ, Hartline CB, Yan Z, Zemlicka J, Quenelle DC. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob Agents Chemother. 2004a;48(12):4745–4753. doi: 10.1128/AAC.48.12.4745-4753.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Quenelle DC. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004b;48(9):3516–3522. doi: 10.1128/AAC.48.9.3516-3522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER, Hartline CB, Rybak RJ, Drach JC, Townsend LB, Biron KK, Bidanset DJ. Activities of benzimidazole D- and L-ribonucleosides in animal models of cytomegalovirus infections. Antimicrob Agents Chemother. 2004c;48(5):1749–1755. doi: 10.1128/AAC.48.5.1749-1755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER, Kushner NL, Hartline CB, Williams-Aziz SL, Harden EA, Zhou S, Zemlicka J, Prichard MN. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob Agents Chemother. 2005;49(3):1039–1045. doi: 10.1128/AAC.49.3.1039-1045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER, Rybak RJ, Hartline CB, Bidanset DJ. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir Chem Chemother. 2001;12(Suppl 1):149–156. [PubMed] [Google Scholar]
- Kimberlin DW, Acosta EP, Sanchez PJ, Sood S, Agrawal V, Homans J, Jacobs RF, Lang D, Romero JR, Griffin J, Cloud GA, Lakeman FD, Whitley RJ National Institute of, A., Infectious Diseases Collaborative Antiviral Study, G. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis. 2008;197(6):836–845. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, Jacobs RF, Vaudry W, Pass RF, Kiell JM, Soong SJ, Whitley RJ National Institute of, A., Infectious Diseases Collaborative Antiviral Study, G. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- Komazin G, Townsend LB, Drach JC. Role of a mutation in human cytomegalovirus gene UL104 in resistance to benzimidazole ribonucleosides. J Virol. 2004;78(2):710–715. doi: 10.1128/JVI.78.2.710-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszalka GW, Johnson NW, Good SS, Boyd L, Chamberlain SC, Townsend LB, Drach JC, Biron KK. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 2002;46(8):2373–2380. doi: 10.1128/AAC.46.8.2373-2380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosky PM, Borysko KZ, Nassiri MR, Devivar RV, Ptak RG, Davis MG, Biron KK, Townsend LB, Drach JC. Phosphorylation of beta-D-ribosylbenzimidazoles is not required for activity against human cytomegalovirus. Antimicrob Agents Chemother. 2002;46(2):478–486. doi: 10.1128/AAC.46.2.478-486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosky PM, Underwood MR, Turk SR, Feng KW, Jain RK, Ptak RG, Westerman AC, Biron KK, Townsend LB, Drach JC. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72(6):4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner NL, Williams SL, Hartline CB, Harden EA, Bidanset DJ, Chen X, Zemlicka J, Kern ER. Efficacy of methylenecyclopropane analogs of nucleosides against herpesvirus replication in vitro. Nucleosides Nucleotides Nucleic Acids. 2003;22(12):2105–2119. doi: 10.1081/ncn-120026633. [DOI] [PubMed] [Google Scholar]
- Lalezari JP, Aberg JA, Wang LH, Wire MB, Miner R, Snowden W, Talarico CL, Shaw S, Jacobson MA, Drew WL. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother. 2002;46(9):2969–2976. doi: 10.1128/AAC.46.9.2969-2976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari JP, Stagg RJ, Kuppermann BD, Holland GN, Kramer F, Ives DV, Youle M, Robinson MR, Drew WL, Jaffe HS. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann Intern Med. 1997;126(4):257–263. doi: 10.7326/0003-4819-126-4-199702150-00001. [DOI] [PubMed] [Google Scholar]
- Leung DT, Sacks SL. Current recommendations for the treatment of genital herpes. Drugs. 2000;60(6):1329–1352. doi: 10.2165/00003495-200060060-00007. [DOI] [PubMed] [Google Scholar]
- Li F, Kenyon KW, Kirby KA, Fishbein DP, Boeckh M, Limaye AP. Incidence and clinical features of ganciclovir-resistant cytomegalovirus disease in heart transplant recipients. Clin Infect Dis. 2007;45(4):439–447. doi: 10.1086/519941. [DOI] [PubMed] [Google Scholar]
- Li SB, Yang ZH, Feng JS, Fong CK, Lucia HL, Hsiung GD. Activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) against guinea pig cytomegalovirus infection in cultured cells and in guinea pigs. Antiviral Res. 1990;13(5):237–252. doi: 10.1016/0166-3542(90)90069-j. [DOI] [PubMed] [Google Scholar]
- Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000;356(9230):645–649. doi: 10.1016/S0140-6736(00)02607-6. [DOI] [PubMed] [Google Scholar]
- Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis. 2002;185(1):20–27. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, Ruebsamen-Schaeff H, Zimmermann H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother. 2010;54(3):1290–1297. doi: 10.1128/AAC.01596-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi PL, Landowski CP, Brancale A, Song X, Townsend LB, Drach JC, Amidon GL. N-methylpurine DNA glycosylase and 8-oxoguanine dna glycosylase metabolize the antiviral nucleoside 2-bromo-5,6-dichloro-1-(beta-D-ribofuranosyl)benzimidazole. Drug Metab Dispos. 2006;34(6):1070–1077. doi: 10.1124/dmd.105.009209. [DOI] [PubMed] [Google Scholar]
- Lurain NS, Bhorade SM, Pursell KJ, Avery RK, Yeldandi VV, Isada CM, Robert ES, Kohn DJ, Arens MQ, Garrity ER, Taege AJ, Mullen MG, Todd KM, Bremer JW, Yen-Lieberman B. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J Infect Dis. 2002;186(6):760–768. doi: 10.1086/342844. [DOI] [PubMed] [Google Scholar]
- Ma JD, Nafziger AN, Villano SA, Gaedigk A, Bertino JS., Jr Maribavir pharmacokinetics and the effects of multiple-dose maribavir on cytochrome P450 (CYP) 1A2, CYP 2C9, CYP 2C19, CYP 2D6, CYP 3A, N-acetyltransferase-2, and xanthine oxidase activities in healthy adults. Antimicrob Agents Chemother. 2006;50(4):1130–1135. doi: 10.1128/AAC.50.4.1130-1135.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfori JE, Exner MM, Marousek GI, Chou S, Drew WL. Development of new cytomegalovirus UL97 and DNA polymerase mutations conferring drug resistance after valganciclovir therapy in allogeneic stem cell recipients. J Clin Virol. 2007;38(2):120–125. doi: 10.1016/j.jcv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Marschall M, Stein-Gerlach M, Freitag M, Kupfer R, van Den Bogaard M, Stamminger T. Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. J Gen Virol. 2001;82(Pt 6):1439–1450. doi: 10.1099/0022-1317-82-6-1439. [DOI] [PubMed] [Google Scholar]
- Marschall M, Stein-Gerlach M, Freitag M, Kupfer R, van den Bogaard M, Stamminger T. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J Gen Virol. 2002;83(Pt 5):1013–1023. doi: 10.1099/0022-1317-83-5-1013. [DOI] [PubMed] [Google Scholar]
- McSharry JJ, McDonough A, Olson B, Hallenberger S, Reefschlaeger J, Bender W, Drusano GL. Susceptibilities of human cytomegalovirus clinical isolates to BAY38–4766, BAY43–9695, and ganciclovir. Antimicrob Agents Chemother. 2001a;45(10):2925–2927. doi: 10.1128/AAC.45.10.2925-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry JJ, McDonough A, Olson B, Talarico C, Davis M, Biron KK. Inhibition of ganciclovir-susceptible and -resistant human cytomegalovirus clinical isolates by the benzimidazole L-riboside 1263W94. Clin Diagn Lab Immunol. 2001b;8(6):1279–1281. doi: 10.1128/CDLI.8.6.1279-1281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVoy MA, Nixon DE. Impact of 2-bromo-5,6-dichloro-1-beta-D-ribofuranosyl benzimidazole riboside and inhibitors of DNA, RNA, and protein synthesis on human cytomegalovirus genome maturation. J Virol. 2005;79(17):11115–11127. doi: 10.1128/JVI.79.17.11115-11127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercorelli B, Sinigalia E, Loregian A, Palu G. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev Med Virol. 2008;18(3):177–210. doi: 10.1002/rmv.558. [DOI] [PubMed] [Google Scholar]
- Mhaske SB, Ksebati B, Prichard MN, Drach JC, Zemlicka J. Phosphonate analogues of cyclopropavir phosphates and their E-isomers. Synthesis and antiviral activity. Bioorg Med Chem. 2009;17(11):3892–3899. doi: 10.1016/j.bmc.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354(20):2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother. 2009;63(5):862–867. doi: 10.1093/jac/dkp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M, Davis C, Boeckh M. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97(4):867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- Painter GR, Hostetler KY. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 2004;22(8):423–427. doi: 10.1016/j.tibtech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Painter GR, Trost LC, Lambert BL, Almond MR, Buller M, Kern ER, Painter GP, Robertson AT, O’Mahony R. CMX001. Drugs of the future. 2008;33(8):1–7. [Google Scholar]
- Pari GS. Nuts and bolts of human cytomegalovirus lytic DNA replication. Curr Top Microbiol Immunol. 2008;325:153–166. doi: 10.1007/978-3-540-77349-8_9. [DOI] [PubMed] [Google Scholar]
- Parker S, Touchette E, Oberle C, Almond M, Robertson A, Trost LC, Lampert B, Painter G, Buller RM. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 2008;77(1):39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrottet N, Decosterd LA, Meylan P, Pascual M, Biollaz J, Buclin T. Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin Pharmacokinet. 2009;48(6):399–418. doi: 10.2165/00003088-200948060-00006. [DOI] [PubMed] [Google Scholar]
- Potena L, Valantine HA. Cytomegalovirus-associated allograft rejection in heart transplant patients. Curr Opin Infect Dis. 2007;20(4):425–431. doi: 10.1097/QCO.0b013e328259c33b. [DOI] [PubMed] [Google Scholar]
- Prichard MN. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev Med Virol. 2009;19(4):215–229. doi: 10.1002/rmv.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Gao N, Jairath S, Mulamba G, Krosky P, Coen DM, Parker BO, Pari GS. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J Virol. 1999;73(7):5663–5670. doi: 10.1128/jvi.73.7.5663-5670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Hartline CB, Harden EA, Daily SL, Beadle JR, Valiaeva N, Kern ER, Hostetler KY. Inhibition of herpesvirus replication by hexadecyloxypropyl esters of purine- and pyrimidine-based phosphonomethoxyethyl nucleoside phosphonates. Antimicrob Agents Chemother. 2008;52(12):4326–4330. doi: 10.1128/AAC.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Quenelle DC, Bidanset DJ, Komazin G, Chou S, Drach JC, Kern ER. Human cytomegalovirus UL27 is not required for viral replication in human tissue implanted in SCID mice. Virol J. 2006;3:18. doi: 10.1186/1743-422X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YL, Geiser F, Kira T, Gullen E, Cheng YC, Ptak RG, Breitenbach JM, Drach JC, Hartline CB, Kern ER, Zemlicka J. Synthesis and enantioselectivity of the antiviral effects of (R,Z)-,(S,Z)-methylenecyclopropane analogues of purine nucleosides and phosphoralaninate prodrugs: influence of heterocyclic base, type of virus and host cells. Antivir Chem Chemother. 2000;11(3):191–202. doi: 10.1177/095632020001100302. [DOI] [PubMed] [Google Scholar]
- Qiu YL, Ksebati MB, Ptak RG, Fan BY, Breitenbach JM, Lin JS, Cheng YC, Kern ER, Drach JC, Zemlicka J. (Z)- and (E)-2-((hydroxymethyl)cyclopropylidene)methyladenine and -guanine. New nucleoside analogues with a broad-spectrum antiviral activity. J Med Chem. 1998a;41(1):10–23. doi: 10.1021/jm9705723. [DOI] [PubMed] [Google Scholar]
- Qiu YL, Ptak RG, Breitenbach JM, Lin JS, Cheng YC, Drach JC, Kern ER, Zemlicka J. Synthesis and antiviral activity of phosphoralaninate derivatives of methylenecyclopropane analogues of nucleosides. Antiviral Res. 1999;43(1):37–53. doi: 10.1016/s0166-3542(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Qiu YL, Ptak RG, Breitenbach JM, Lin JS, Cheng YC, Kern ER, Drach JC, Zemlicka J. (Z)- and (E)-2-(hydroxymethylcyclopropylidene)-methylpurines and pyrimidines as antiviral agents. Antivir Chem Chemother. 1998b;9(4):341–352. [PubMed] [Google Scholar]
- Quenelle DC, Collins DJ, Herrod BP, Keith KA, Trahan J, Beadle JR, Hostetler KY, Kern ER. Effect of oral treatment with hexadecyloxypropyl-[(S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine] [(S)-HPMPA] or octadecyloxyethyl-(S)-HPMPA on cowpox or vaccinia virus infections in mice. Antimicrob Agents Chemother. 2007a;51(11):3940–3947. doi: 10.1128/AAC.00184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Collins DJ, Kern ER. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob Agents Chemother. 2003;47(10):3275–3280. doi: 10.1128/AAC.47.10.3275-3280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Collins DJ, Pettway LR, Hartline CB, Beadle JR, Wan WB, Hostetler KY, Kern ER. Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus (MCMV) or human cytomegalovirus (HCMV) in animal models. Antiviral Res. 2008;79(2):133–135. doi: 10.1016/j.antiviral.2008.01.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Kern ER. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48(2):404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010 doi: 10.1086/656717. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Prichard MN, Keith KA, Hruby DE, Jordan R, Painter GR, Robertson A, Kern ER. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007b;51(11):4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa P, Farasati NA, Shapiro R, Hostetler KY. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob Agents Chemother. 2006;50(4):1564–1566. doi: 10.1128/AAC.50.4.1564-1566.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reefschlaeger J, Bender W, Hallenberger S, Weber O, Eckenberg P, Goldmann S, Haerter M, Buerger I, Trappe J, Herrington JA, Haebich D, Ruebsamen-Waigmann H. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38–4766): in vitro and in vivo antiviral activity and mechanism of action. J Antimicrob Chemother. 2001;48(6):757–767. doi: 10.1093/jac/48.6.757. [DOI] [PubMed] [Google Scholar]
- Rosenthal LS, Fowler KB, Boppana SB, Britt WJ, Pass RF, Schmid SD, Stagno S, Cannon MJ. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J. 2009;28(6):515–520. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Fowler KB, Ashrith G, Stagno S, Britt WJ, Pass RF, Boppana SB. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148(3):332–336. doi: 10.1016/j.jpeds.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ruiz JC, Aldern KA, Beadle JR, Hartline CB, Kern ER, Hostetler KY. Synthesis and antiviral evaluation of alkoxyalkyl esters of phosphonopropoxymethyl-guanine and phosphonopropoxymethyl-diaminopurine. Antivir Chem Chemother. 2006;17(2):89–95. doi: 10.1177/095632020601700204. [DOI] [PubMed] [Google Scholar]
- Ruiz JC, Beadle JR, Aldern KA, Keith KA, Hartline CB, Kern ER, Hostetler KY. Synthesis and antiviral evaluation of alkoxyalkyl-phosphate conjugates of cidofovir and adefovir. Antiviral Res. 2007;75(1):87–90. doi: 10.1016/j.antiviral.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak RJ, Hartline CB, Qiu YL, Zemlicka J, Harden E, Marshall G, Sommadossi JP, Kern ER. In vitro activities of methylenecyclopropane analogues of nucleosides and their phosphoralaninate prodrugs against cytomegalovirus and other herpesvirus infections. Antimicrob Agents Chemother. 2000;44(6):1506–1511. doi: 10.1128/aac.44.6.1506-1511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak RJ, Zemlicka J, Qiu YL, Hartline CB, Kern ER. Effective treatment of murine cytomegalovirus infections with methylenecyclopropane analogues of nucleosides. Antiviral Res. 1999;43(3):175–188. doi: 10.1016/s0166-3542(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Safrin S, Cherrington J, Jaffe HS. Clinical uses of cidofovir. Rev Med Virol. 1997;7(3):145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Scott GM, Weinberg A, Rawlinson WD, Chou S. Multidrug resistance conferred by novel DNA polymerase mutations in human cytomegalovirus isolates. Antimicrob Agents Chemother. 2007;51(1):89–94. doi: 10.1128/AAC.00633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck R, Andrei G, Bodaghi B, Lagneaux L, Daelemans D, de Clercq E, Neyts J, Schols D, Naesens L, Michelson S, Bron D, Otto MJ, Bousseau A, Nemecek C, Roy C. 2-Chloro-3-pyridin-3-yl-5,6,7,8-tetrahydroindolizine-1-carboxamide (CMV423), a new lead compound for the treatment of human cytomegalovirus infections. Antiviral Res. 2002;55(3):413–424. doi: 10.1016/s0166-3542(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Snoeck R, De Clercq E. Role of cidofovir in the treatment of DNA virus infections, other than CMV infections, in immunocompromised patients. Curr Opin Investig Drugs. 2002;3(11):1561–1566. [PubMed] [Google Scholar]
- Snoeck R, Sakuma T, De Clercq E, Rosenberg I, Holy A. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 1988;32(12):1839–1844. doi: 10.1128/aac.32.12.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokos DR, Berger M, Lazarus HM. Intravenous immunoglobulin: appropriate indications and uses in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(3):117–130. doi: 10.1053/bbmt.2002.v8.pm11939601. [DOI] [PubMed] [Google Scholar]
- Stagno S. Cytomegalovirus. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. 5. Saunders; Philadelphia: 2001. p. xiv.p. 1507. [Google Scholar]
- Stals FS, de Clercq E, Bruggeman CA. Comparative activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine against rat cytomegalovirus infection in vitro and in vivo. Antimicrob Agents Chemother. 1991;35(11):2262–2266. doi: 10.1128/aac.35.11.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V, Talarico CL, Stanat SC, Davis M, Coen DM, Biron KK. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;359(6390):85. doi: 10.1038/359085a0. [DOI] [PubMed] [Google Scholar]
- Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis. 2008;47(5):702–711. doi: 10.1086/590934. [DOI] [PubMed] [Google Scholar]
- Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR, Wold WS. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci U S A. 2008;105(20):7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend LB, Devivar RV, Turk SR, Nassiri MR, Drach JC. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J Med Chem. 1995;38(20):4098–4105. doi: 10.1021/jm00020a025. [DOI] [PubMed] [Google Scholar]
- Underwood MR, Ferris RG, Selleseth DW, Davis MG, Drach JC, Townsend LB, Biron KK, Boyd FL. Mechanism of action of the ribopyranoside benzimidazole GW275175X against human cytomegalovirus. Antimicrob Agents Chemother. 2004;48(5):1647–1651. doi: 10.1128/AAC.48.5.1647-1651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MR, Harvey RJ, Stanat SC, Hemphill ML, Miller T, Drach JC, Townsend LB, Biron KK. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72(1):717–725. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiaeva N, Beadle JR, Aldern KA, Trahan J, Hostetler KY. Synthesis and antiviral evaluation of alkoxyalkyl esters of acyclic purine and pyrimidine nucleoside phosphonates against HIV-1 in vitro. Antiviral Res. 2006;72(1):10–19. doi: 10.1016/j.antiviral.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Valiaeva N, Prichard MN, Buller RM, Beadle JR, Hartline CB, Keith KA, Schriewer J, Trahan J, Hostetler KY. Antiviral evaluation of octadecyloxyethyl esters of (S)-3-hydroxy-2-(phosphonomethoxy)propyl nucleosides against herpesviruses and orthopoxviruses. Antiviral Res. 2009;84(3):254–259. doi: 10.1016/j.antiviral.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff AJ, Bryson HM. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48(2):199–226. doi: 10.2165/00003495-199448020-00007. [DOI] [PubMed] [Google Scholar]
- Walmsley SL, Chew E, Read SE, Vellend H, Salit I, Rachlis A, Fanning MM. Treatment of cytomegalovirus retinitis with trisodium phosphonoformate hexahydrate (Foscarnet) J Infect Dis. 1988;157(3):569–572. doi: 10.1093/infdis/157.3.569. [DOI] [PubMed] [Google Scholar]
- Wan WB, Beadle JR, Hartline C, Kern ER, Ciesla SL, Valiaeva N, Hostetler KY. Comparison of the antiviral activities of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 2005;49(2):656–662. doi: 10.1128/AAC.49.2.656-662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen MW. Non-nucleoside inhibitors of herpesviruses. Rev Med Virol. 2002;12(3):167–178. doi: 10.1002/rmv.354. [DOI] [PubMed] [Google Scholar]
- Williams-Aziz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, Beadle JR, Wan WB, Hostetler KY, Kern ER. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother. 2005;49(9):3724–3733. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SL, Hartline CB, Kushner NL, Harden EA, Bidanset DJ, Drach JC, Townsend LB, Underwood MR, Biron KK, Kern ER. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob Agents Chemother. 2003;47(7):2186–2192. doi: 10.1128/AAC.47.7.2186-2192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Drach JC, Prichard MN, Yanachkova M, Yanachkov I, Bowlin TL, Zemlicka J. l-Valine ester of cyclopropavir: a new antiviral prodrug. Antivir Chem Chemother. 2009;20(1):37–46. doi: 10.3851/IMP782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant. 2004;4(8):1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- Zemlicka J. Allenols Derived from Nucleic Acids Bases – A New Class of Anti-HIV Agents: Chemistry and Biological Activity. In: Chu CK, Baker DC, editors. Nucleosides and nucleotides as antitumor and antiviral agents. Plenum Press; New York: 1993. p. x.p. 336. [Google Scholar]
- Zemlicka J. Unusual Analogues of Nucleosides: Chemistry and Biological Activity. In: Chu CK, editor. Recent advances in nucleosides : chemistry and chemotherapy. 1. Elsevier; Amsterdam; Boston, Mass: 2002. p. ix.p. 533. [Google Scholar]
- Zhou S, Breitenbach JM, Borysko KZ, Drach JC, Kern ER, Gullen E, Cheng YC, Zemlicka J. Synthesis and antiviral activity of (Z)- and (E)-2,2-[bis(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines: second-generation methylenecyclopropane analogues of nucleosides. J Med Chem. 2004a;47(3):566–575. doi: 10.1021/jm030316s. [DOI] [PubMed] [Google Scholar]
- Zhou S, Drach JC, Prichard MN, Zemlicka J. (Z)- and (E)-2-(1,2-dihydroxyethyl)methylenecyclopropane analogues of 2′-deoxyadenosine and 2′-deoxyguanosine. Synthesis of all stereoisomers, absolute configuration, and antiviral activity. J Med Chem. 2009;52(10):3397–3407. doi: 10.1021/jm900126v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kern ER, Gullen E, Cheng YC, Drach JC, Matsumi S, Mitsuya H, Zemlicka J. (Z)-and (E)-[2-Fluoro-2-(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines, a new class of methylenecyclopropane analogues of nucleosides: synthesis and antiviral activity. J Med Chem. 2004b;47(27):6964–6972. doi: 10.1021/jm040093l. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zemlicka J. Synthesis of 2,2,3-tris(hydroxymethyl)methylenecyclopropane analogues of nucleosides. Nucleosides Nucleotides Nucleic Acids. 2007;26(4):391–402. doi: 10.1080/15257770701297000. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zemlicka J, Kern ER, Drach JC. Fluoroanalogues of anti-cytomegalovirus agent cyclopropavir: synthesis and antiviral activity of (E)- and (Z)-9-{[2,2-bis(hydroxymethyl)-3-fluorocyclopropylidene]methyl}-adenines and guanines. Nucleosides Nucleotides Nucleic Acids. 2007;26(3):231–243. doi: 10.1080/15257770701257210. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Wilts H, Lenhardt M, Hahn M, Mertens T. Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase. Antiviral Res. 2000;48(1):49–60. doi: 10.1016/s0166-3542(00)00118-2. [DOI] [PubMed] [Google Scholar]