Figure 5. Anti-CCL2 mAb administration in combination with vaccines targeting glioma-associated antigens (GAAs).
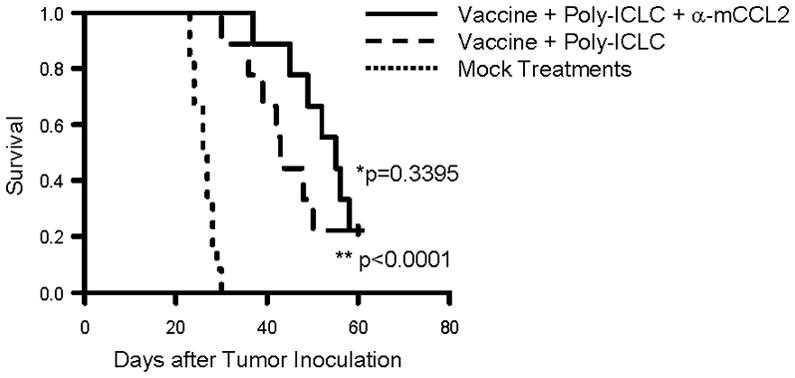
C57BL/6 mice bearing GL261 glioma received subcutaneous immunizations with 100 μg of HBV core128–140 (TPPAYRPPNAPIL) T-helper epitope peptide and GAA peptides, 100 μg each of H-2Db-binding mEphA2671–679 (FSHHNIIRL), H-2Db –binding mGARC-177–85 (AALLNKLYA), H-2Db-binding human gp100 (hgp100)25–33 (KVPRNQDWL), H-2Kb-binding mEphA2682–689 (VVSKYKPM), H-2Kb–binding mTRP2180–188 (SVYDFFVWL) peptide emulsified in Incomplete Freund Adjuvant (IFA) on days 2, 12 and 22 after tumor inoculation. In addition, poly-ICLC (20 μg/injection in 20 μl) was intramuscularly injected twice a week starting on day 2 till day 30 after tumor inoculation (n=18). Nine of 18 immunized mice were also treated with anti-mouse CCL2 mAb (2 mg/kg/dose) by i.p. injections starting on day 7, twice weekly up to 8 weeks after tumor cell inoculation (2 mg/kg/dose). Twelve control mice received mock vaccines consisted of 100 μg of HBV core128–140 but without GAA-peptides emulsified in IFA and control IgG (2 mg/kg/dose) was injected i.p. twice a week starting on day 7 for total 8 weeks after tumor inoculation. SFS of mice was monitored. (**p < 0.0001 for poly-ICLC-assisted GAA-vaccine plus anti-CCL2 mAb vs. mock-vaccines and control IgG; *p = 0.3395 for poly-ICLC-assisted GAA-vaccine plus anti-CCL2 mAb vs. poly-ICLC-assisted GAA-vaccine and isotype IgG).
