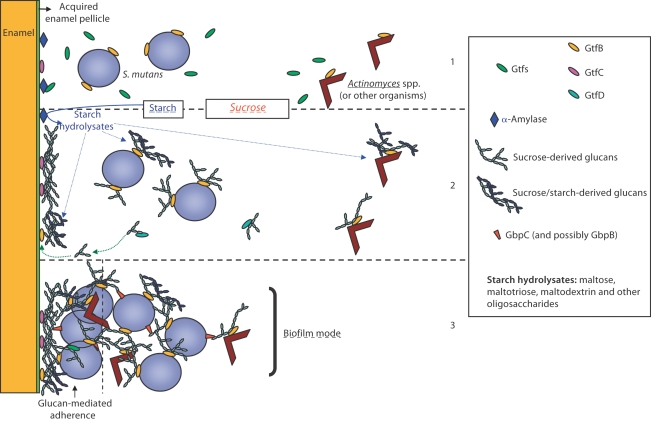
Fig. 2.
Revised model of Gtf-glucan-mediated bacterial adherence and cariogenic biofilm development. Originally proposed by Rölla et al. [1983b]. (1) The Gtfs secreted by S. mutans are incorporated into pellicle (particularly GtfC) and adsorb on bacterial surfaces (mainly GtfB), including microorganisms that do not produce Gtfs (e.g. Actinomyces spp.). Furthermore, salivary α-amylase is also included into pellicle, which can also bind Gtfs. (2) Surface-adsorbed GtfB and GtfC rapidly utilize dietary sucrose to synthesize insoluble and soluble glucans in situ; the soluble glucans formed by GtfD could serve as primers for GtfB enhancing the overall synthesis of exopolysaccharides. Concomitantly, starch is digested by amylase releasing maltose and a myriad of oligosaccharides; they can be incorporated into the polymer molecule through acceptor reactions, particularly by surface-adsorbed GtfB. The Gtfs adsorbed onto enamel and microbial surfaces provide in situ an insoluble matrix for dental plaque. (3) The glucan molecules provide avid binding sites on surfaces for S. mutans (and other microorganisms) mediating tight bacterial clustering and adherence to the tooth enamel. Furthermore, Gtf-adsorbed bacteria become de facto glucan producers binding to tooth and microbial surfaces by the same mechanisms. This model could explain the rapid formation and accumulation of highly cohesive-adherent plaque in the presence of sucrose (and possibly starch) even if the number of S. mutans is relatively low. After the establishment of a glucan-rich biofilm matrix, ecological pressure (e.g. pH) will determine which bacteria may survive and dominate within plaque under frequent sucrose (or other fermentable carbohydrate) exposure.