Abstract
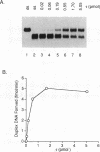
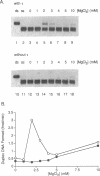
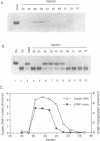
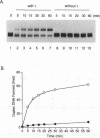
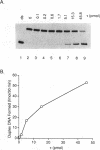
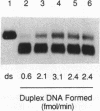
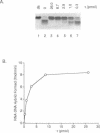
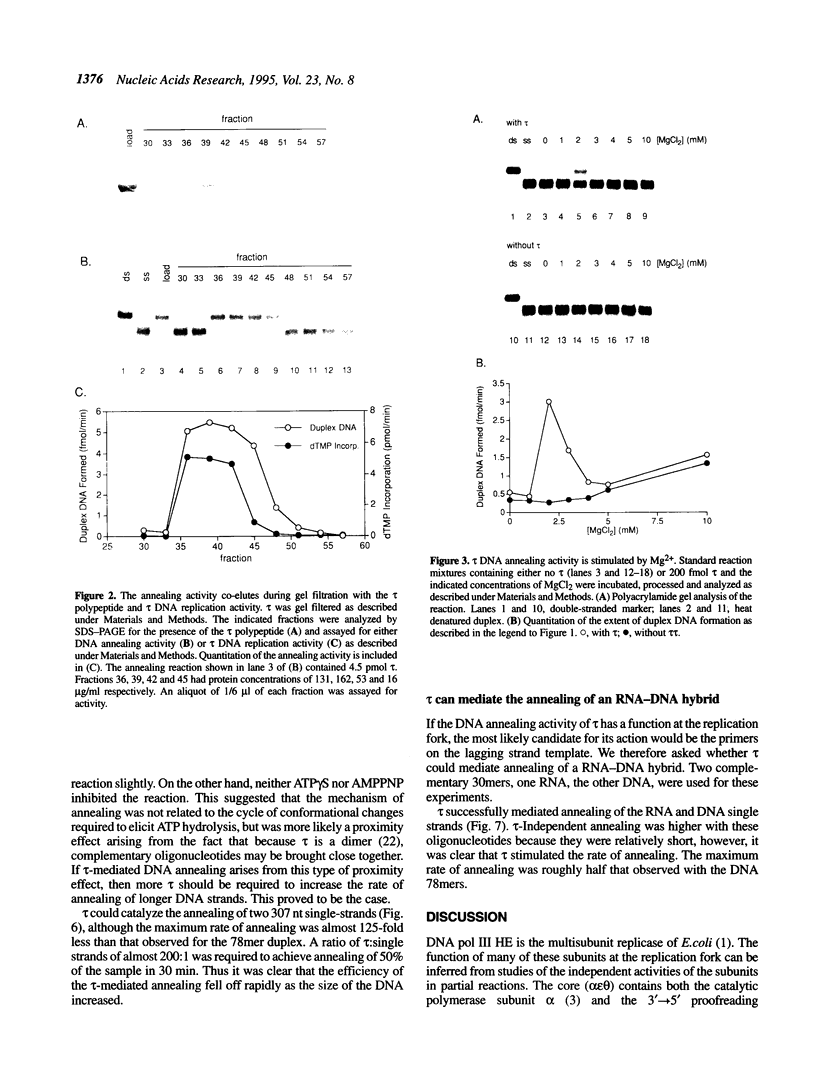
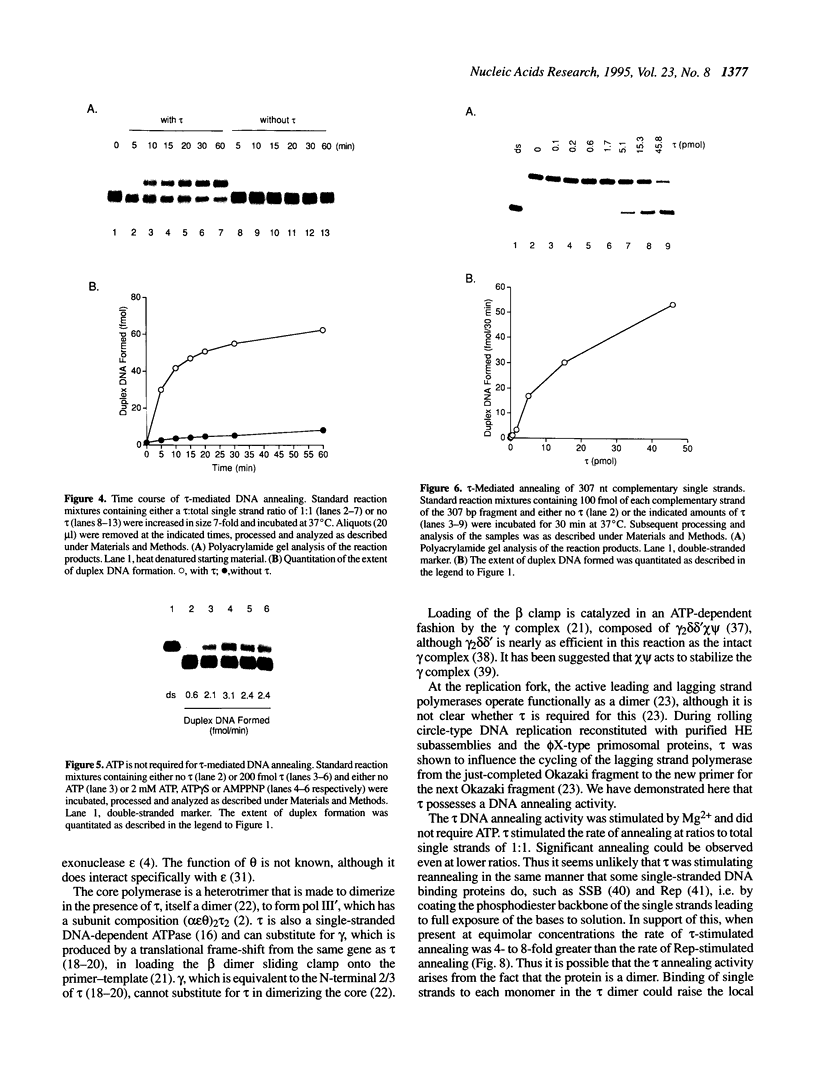
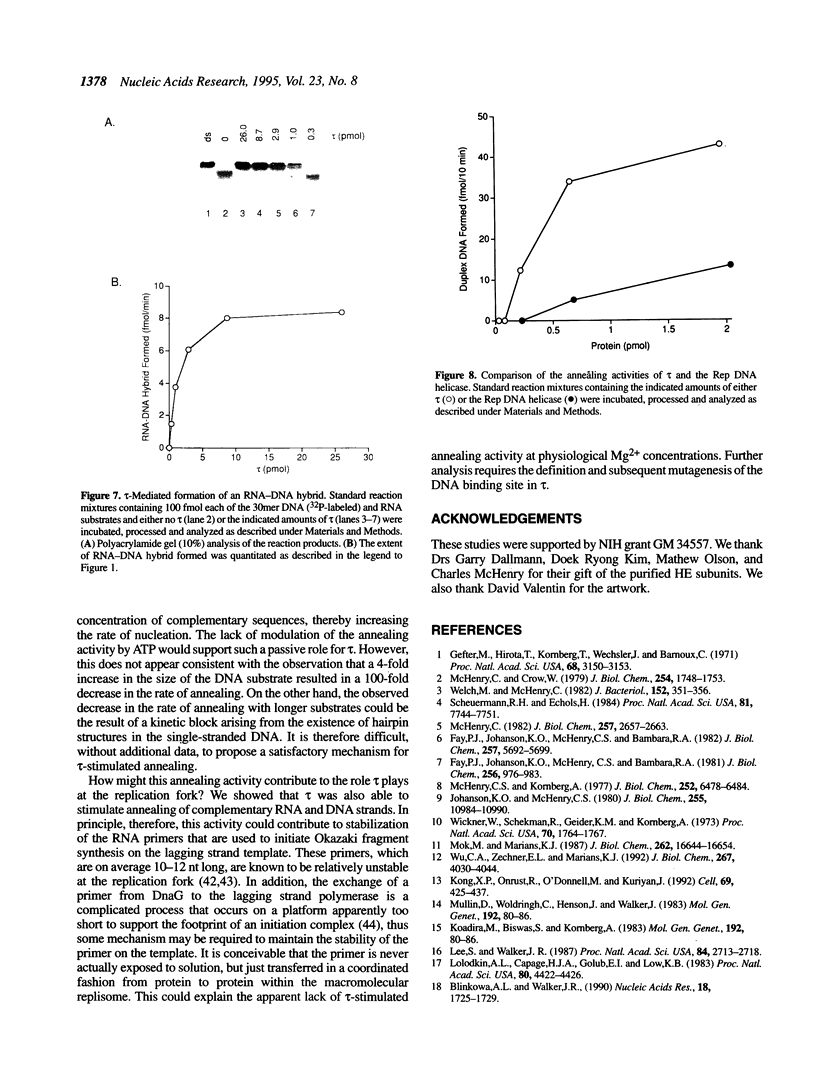
The DNA polymerase III (pol III)holoenzyme is the 10 subunit replicase of Escherichia coli. The 71 kDa tau subunit, encoded by dnaX, dimerizes the core polymerase (alpha epsilon theta) to form pol III'[(alpha epsilon theta)2 tau 2]. tau is also a single-stranded DNA-dependent ATPase and can substitute for the gamma subunit during initiation complex formation. We show here that tau also possesses a DNA-DNA and RNA-DNA annealing activity that is stimulated by Mg2+, but neither requires ATP nor is inhibited by non-hydrolyzable ATP analogs. This suggests the tau may act to stabilize the primer-template interaction during DNA replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai N., Arai K., Kornberg A. Complexes of Rep protein with ATP and DNA as a basis for helicase action. J Biol Chem. 1981 May 25;256(10):5287–5293. [PubMed] [Google Scholar]
- Blinkowa A. L., Walker J. R. Programmed ribosomal frameshifting generates the Escherichia coli DNA polymerase III gamma subunit from within the tau subunit reading frame. Nucleic Acids Res. 1990 Apr 11;18(7):1725–1729. doi: 10.1093/nar/18.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Franden M. A., Aebersold R., Kim D. R., McHenry C. S. Isolation, sequencing and overexpression of the gene encoding the theta subunit of DNA polymerase III holoenzyme. Nucleic Acids Res. 1993 Jul 11;21(14):3281–3286. doi: 10.1093/nar/21.14.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Franden M. A., Aebersold R., McHenry C. S. Identification, isolation, and characterization of the structural gene encoding the delta' subunit of Escherichia coli DNA polymerase III holoenzyme. J Bacteriol. 1993 Jun;175(12):3812–3822. doi: 10.1128/jb.175.12.3812-3822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Franden M. A., Aebersold R., McHenry C. S. Identification, isolation, and overexpression of the gene encoding the psi subunit of DNA polymerase III holoenzyme. J Bacteriol. 1993 Sep;175(17):5604–5610. doi: 10.1128/jb.175.17.5604-5610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Franden M. A., Aebersold R., McHenry C. S. Molecular cloning, sequencing, and overexpression of the structural gene encoding the delta subunit of Escherichia coli DNA polymerase III holoenzyme. J Bacteriol. 1992 Nov;174(21):7013–7025. doi: 10.1128/jb.174.21.7013-7025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Franden M. A., Lippincott J. A., McHenry C. S. Identification, molecular cloning and characterization of the gene encoding the chi subunit of DNA polymerase III holoenzyme of Escherichia coli. Mol Gen Genet. 1993 Nov;241(3-4):399–408. doi: 10.1007/BF00284693. [DOI] [PubMed] [Google Scholar]
- Dong Z., Onrust R., Skangalis M., O'Donnell M. DNA polymerase III accessory proteins. I. holA and holB encoding delta and delta'. J Biol Chem. 1993 Jun 5;268(16):11758–11765. [PubMed] [Google Scholar]
- Fay P. J., Johanson K. O., McHenry C. S., Bambara R. A. Size classes of products synthesized processively by DNA polymerase III and DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1981 Jan 25;256(2):976–983. [PubMed] [Google Scholar]
- Fay P. J., Johanson K. O., McHenry C. S., Bambara R. A. Size classes of products synthesized processively by two subassemblies of Escherichia coli DNA polymerase III holoenzyme. J Biol Chem. 1982 May 25;257(10):5692–5699. [PubMed] [Google Scholar]
- Flower A. M., McHenry C. S. The gamma subunit of DNA polymerase III holoenzyme of Escherichia coli is produced by ribosomal frameshifting. Proc Natl Acad Sci U S A. 1990 May;87(10):3713–3717. doi: 10.1073/pnas.87.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin L. G., Kornberg A. Prereplicative complexes of components of DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1992 May 25;267(15):10318–10322. [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson K. O., McHenry C. S. Purification and characterization of the beta subunit of the DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10984–10990. [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Kodaira M., Biswas S. B., Kornberg A. The dnaX gene encodes the DNA polymerase III holoenzyme tau subunit, precursor of the gamma subunit, the dnaZ gene product. Mol Gen Genet. 1983;192(1-2):80–86. doi: 10.1007/BF00327650. [DOI] [PubMed] [Google Scholar]
- Kodaira M., Biswas S. B., Kornberg A. The dnaX gene encodes the DNA polymerase III holoenzyme tau subunit, precursor of the gamma subunit, the dnaZ gene product. Mol Gen Genet. 1983;192(1-2):80–86. doi: 10.1007/BF00327650. [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L., Capage M. A., Golub E. I., Low K. B. F sex factor of Escherichia coli K-12 codes for a single-stranded DNA binding protein. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4422–4426. doi: 10.1073/pnas.80.14.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X. P., Onrust R., O'Donnell M., Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992 May 1;69(3):425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. Site-specific recognition of bacteriophage T4 DNA by T4 type II DNA topoisomerase and Escherichia coli DNA gyrase. J Biol Chem. 1984 Apr 25;259(8):5339–5346. [PubMed] [Google Scholar]
- Lee S. H., Walker J. R. Escherichia coli DnaX product, the tau subunit of DNA polymerase III, is a multifunctional protein with single-stranded DNA-dependent ATPase activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2713–2717. doi: 10.1073/pnas.84.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- McHenry C. S. Purification and characterization of DNA polymerase III'. Identification of tau as a subunit of the DNA polymerase III holoenzyme. J Biol Chem. 1982 Mar 10;257(5):2657–2663. [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Minden J. S., Marians K. J. Replication of pBR322 DNA in vitro with purified proteins. Requirement for topoisomerase I in the maintenance of template specificity. J Biol Chem. 1985 Aug 5;260(16):9316–9325. [PubMed] [Google Scholar]
- Mok M., Marians K. J. The Escherichia coli preprimosome and DNA B helicase can form replication forks that move at the same rate. J Biol Chem. 1987 Dec 5;262(34):16644–16654. [PubMed] [Google Scholar]
- O'Donnell M., Studwell P. S. Total reconstitution of DNA polymerase III holoenzyme reveals dual accessory protein clamps. J Biol Chem. 1990 Jan 15;265(2):1179–1187. [PubMed] [Google Scholar]
- Onrust R., O'Donnell M. DNA polymerase III accessory proteins. II. Characterization of delta and delta'. J Biol Chem. 1993 Jun 5;268(16):11766–11772. [PubMed] [Google Scholar]
- Reems J. A., McHenry C. S. Escherichia coli DNA polymerase III holoenzyme footprints three helical turns of its primer. J Biol Chem. 1994 Dec 30;269(52):33091–33096. [PubMed] [Google Scholar]
- Scheuermann R. H., Echols H. A separate editing exonuclease for DNA replication: the epsilon subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studwell-Vaughan P. S., O'Donnell M. Constitution of the twin polymerase of DNA polymerase III holoenzyme. J Biol Chem. 1991 Oct 15;266(29):19833–19841. [PubMed] [Google Scholar]
- Studwell-Vaughan P. S., O'Donnell M. DNA polymerase III accessory proteins. V. Theta encoded by holE. J Biol Chem. 1993 Jun 5;268(16):11785–11791. [PubMed] [Google Scholar]
- Tougu K., Peng H., Marians K. J. Identification of a domain of Escherichia coli primase required for functional interaction with the DnaB helicase at the replication fork. J Biol Chem. 1994 Feb 11;269(6):4675–4682. [PubMed] [Google Scholar]
- Tsuchihashi Z., Kornberg A. Translational frameshifting generates the gamma subunit of DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2516–2520. doi: 10.1073/pnas.87.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M. M., McHenry C. S. Cloning and identification of the product of the dnaE gene of Escherichia coli. J Bacteriol. 1982 Oct;152(1):351–356. doi: 10.1128/jb.152.1.351-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Schekman R., Geider K., Kornberg A. A new form of DNA polymerase 3 and a copolymerase replicate a long, single-stranded primer-template. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Zechner E. L., Hughes A. J., Jr, Franden M. A., McHenry C. S., Marians K. J. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. IV. Reconstitution of an asymmetric, dimeric DNA polymerase III holoenzyme. J Biol Chem. 1992 Feb 25;267(6):4064–4073. [PubMed] [Google Scholar]
- Wu C. A., Zechner E. L., Marians K. J. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. I. Multiple effectors act to modulate Okazaki fragment size. J Biol Chem. 1992 Feb 25;267(6):4030–4044. [PubMed] [Google Scholar]
- Xiao H., Crombie R., Dong Z., Onrust R., O'Donnell M. DNA polymerase III accessory proteins. III. holC and holD encoding chi and psi. J Biol Chem. 1993 Jun 5;268(16):11773–11778. [PubMed] [Google Scholar]
- Xiao H., Dong Z., O'Donnell M. DNA polymerase III accessory proteins. IV. Characterization of chi and psi. J Biol Chem. 1993 Jun 5;268(16):11779–11784. [PubMed] [Google Scholar]
- Zechner E. L., Wu C. A., Marians K. J. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. II. Frequency of primer synthesis and efficiency of primer utilization control Okazaki fragment size. J Biol Chem. 1992 Feb 25;267(6):4045–4053. [PubMed] [Google Scholar]
- Zechner E. L., Wu C. A., Marians K. J. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. III. A polymerase-primase interaction governs primer size. J Biol Chem. 1992 Feb 25;267(6):4054–4063. [PubMed] [Google Scholar]