INTRODUCTION
The risk of peripartum HIV transmission can be reduced from 25% to 12% by the administration of a single dose of nevirapine to an HIV-infected, pregnant woman in labor and to her infant at birth.1 More effective antiretroviral regimens for the prevention of mother-to-child transmission are now recommended,2,3 and access to such regimens is steadily improving.4 However, in settings with incomplete access to comprehensive ART, or when women are ART ineligible, ease of administration and low cost have resulted in the use of single-dose nevirapine (sdNVP) by at least 1.5 million women worldwide, including at least 340,000 women in South Africa.2,5,6
Exposure to sdNVP may result in HIV mutations which confer resistance to nevirapine and efavirenz, two non-nucleoside reverse transcriptase inhibitors (NNRTIs) that form the basis of 1st-line antiretroviral therapy (ART) in most resource-limited settings.7 As a result, sdNVP exposure may reduce the effectiveness of the antiretroviral regimen most likely to be available when women later require therapy for their own HIV disease.2,5,8,9 The OCTANE trial randomized women with screening CD4 <200/μL, with sdNVP exposure (Trial 1) and without sdNVP exposure (Trial 2), to initiate either nevirapine- or lopinavir/ritonavir-based ART. Among sdNVP-exposed women (Trial 1), the lopinavir/ritonavir arm demonstrated significantly lower rates of virologic failure or death than the nevirapine arm.10 Revised 2010 WHO treatment guidelines now recommend protease inhibitor-based ART for women initiating ART within 12 months of sdNVP exposure.11 However, because lopinavir/ritonavir is currently 12 times more expensive than nevirapine,12 policy makers must consider whether lopinavir/ritonavir provides sufficient health benefits in 1st-line ART to warrant its additional cost. Our objective was to evaluate the cost-effectiveness of 1st-line lopinavir/ritonavir- compared to 1st-line nevirapine-based ART in a cohort of women with prior sdNVP exposure.
METHODS
Overview
We used a published computer simulation model of HIV infection,13 with data from OCTANE Trial 110 and cohorts in South Africa,14,15 to project the outcomes of initial antiretroviral regimens among sdNVP-exposed women. In addition to a No ART strategy, we evaluated the two specific ART strategies examined in the OCTANE trial: 1st-line nevirapine followed by 2nd-line lopinavir/ritonavir, and 1st-line lopinavir/ritonavir followed by 2nd-line nevirapine.
For each strategy, we projected life expectancy, opportunistic infections, and HIV-related healthcare costs. Outcomes were evaluated in the short term (2–5 years) and over patient lifetimes to calculate cost-effectiveness ratios of alternative ART strategies. Incremental cost-effectiveness ratios were expressed in 2008 US$/year of life saved (YLS), with costs converted from South African Rand (ZAR) at the 2008 average exchange rate (1 USD = 8.2612 ZAR).16 All costs and health benefits were discounted at 3% annually.17 Assuming a modified societal perspective, patients’ costs for time, transportation, and child care were excluded.17 The World Health Organization (WHO) suggests that health interventions be considered “very cost-effective” if their cost per quality-adjusted life year is less than a country’s per-capita gross domestic product18 (GDP: $5,700 for South Africa in 200819). Due to limited HIV-related quality-of-life data in Africa, we computed cost-effectiveness ratios in $/YLS rather than in $/quality-adjusted YLS, thereby using WHO thresholds as general guidance only.
Model
The Cost-effectiveness of Preventing AIDS Complications (CEPAC)-International model for South Africa is a first-order Monte Carlo simulation of HIV infection (Supplementary Appendix).13,20 HIV-infected women are simulated individually from model entry through death. Disease progression is characterized by a sequence of monthly transitions between health states; these include acute opportunistic and other infections prevalent in South Africa, chronic HIV infection, and death. The model records all clinical events and costs during each patient’s lifetime. A cohort of one million women is simulated to produce stable estimates of outcomes.
In the absence of effective ART, current HIV RNA level determines the modeled rate of CD4 cell decline (Table 1). Current CD4 count, opportunistic infection (OI) prophylaxis, and history or absence of previous OIs determine the risk of OIs and HIV-related death. HIV RNA suppression with effective ART leads CD4 counts to increase, reducing the risks for OIs and death. Virologic failure on ART may occur either “early” (<24 weeks) or “late” (>24 weeks) after ART initiation. The resulting rise in HIV RNA reflects “true” virologic failure for simulated patients, and is distinct from the ability to detect “observed” failure through HIV RNA monitoring. For patients with early or late virologic failure, CD4 counts decline, accompanied by increased risks of OIs and death.
Table 1.
Selected clinical parameters used in a computer simulation of antiretroviral therapy strategies following exposure to single-dose nevirapine.
| Variable | Base Case Value | Data sources |
|---|---|---|
|
Baseline cohort characteristics |
OCTANE Trial 110 | |
| Age (mean (SD), in years) | 31 (5) | |
| CD4 count (mean (SD),/μL) | 135 (61) | |
| Distribution of initial HIV RNA (% total) | ||
| >100,000 copies/ml | 63 | |
| 30,001–100,000 copies/ml | 23 | |
| 10,001–30,000 copies/ml | 9 | |
| 3,001–10,000 copies/ml | 4 | |
| 501–3,000 copies/ml | 2 | |
| ≤ 500 copies/ml | 0 | |
| Time from sdNVP exposure to ART initiation (median (10th, 90th percentile), in months) | 17 (7, 45) | |
| Proportion (%) with detectable NNRTI resistance (Viroseq standard genotype assay) at baseline | 14 | |
|
Natural history of disease | ||
| Mean monthly decrease in CD4/μL by HIV RNA | Multicenter AIDS Cohort Study47 | |
| >30,000 copies/ml | 6.4 | |
| 10,001–30,000 copies/ml | 5.4 | |
| 3,001–10,000 copies/ml | 4.6 | |
| 501–3,000 copies/ml | 3.7 | |
| 0–500 copies/ml | 3.0 | |
| Monthly risk of severe opportunistic infections (%, range by CD4 count) | Cape Town AIDS Cohort14 | |
| Severe bacterial infection | 0.08–0.71 | |
| Severe fungal infection | 0.02–2.22 | |
| Toxoplasmosis | 0.00–0.06 | |
| Pneumocystis jiroveci pneumonia | 0.00–0.12 | |
| Mycobacterium avium | 0.00–0.30 | |
| Other WHO stage 3–4 condition | 0.25–2.57 | |
| Monthly risk of other clinical conditions (%, range by CD4 count) | Cape Town AIDS Cohort14 | |
| Mild fungal infection | 0.59–3.51 | |
| Tuberculosis (pulmonary or extrapulmonary) | 0.21–1.96 | |
| Herpes simplex, varicella zoster, other mucocutaneous manifestions of HIV | 2.51–3.11 | |
| Monthly risk of HIV-related death (%, range by CD4 count) | Cape Town AIDS Cohort14 | |
| No history of opportunistic infection | 0.09–3.33 | |
| With history of opportunistic infection | 0.30–7.94 | |
|
Antiretroviral therapy | ||
| Efficacy (% HIV RNA suppression at 24 weeks; gain in CD4/μL at 24 weeks on suppressive ARTa (assumed equal to entire cohort for all subgroups and for 2nd-line ART); yearly risk (%) of virologic failure >24 weeks after initiation) | ||
| 1st-line NVP/TDF/FTC | OCTANE Trial 110 | |
| Entire OCTANE cohort (base case) | 85%; 173; 10.61 | |
| No detectable NNRTI resistance at baseline | 89%; 173; 5.95 | |
| Detectable NNRTI resistance at baseline | 60%; 173; 49.41 | |
| ART initiation 6–12 months after sdNVP | 82%; 173; 21.34 | |
| ART initiation 12–24 months after sdNVP | 81%; 173; 7.42 | |
| ART initiation >24 months after sdNVP | 91%; 173; 10.61b | |
| 1st-line LPV/r/TDF/FTC | OCTANE Trial 110 | |
| Entire OCTANE cohort (base case) | 97%; 162; 2.84 | |
| No detectable NNRTI resistance at baseline | 97%; 162; 3.19 | |
| Detectable NNRTI resistance at baseline | 94%; 162; 2.84 b | |
| ART initiation 6–12 months after sdNVP | 97%; 162; 2.84 b | |
| ART initiation 12–24 months after sdNVP | 98%; 162; 3.19 | |
| ART initiation >24 months after sdNVP | 94%; 162; 5.72 | |
| 2nd-line NVP/ddI/ZDV (base case) | 43%; 173; 10.61 | UK, SAfr;26,27 late failure assumed = 1st-line nevirapine |
| 2nd line LPV/r/ddI/ZDV (base case) | 72%; 162; 2.84 | Malawi, SAfr, Uganda;27,28,36,late failure assumed = 1st-line LPV/r |
| 3rd line DRV/r/3TC (sensitivity analysis) | 45%; 162; 29.84 | POWER;33 assumption (CD4 gain = 1st-line LPV/r) |
| LPV/r/3TC after failure of 2nd line ART | 12%; 18 (CD4 gain 1st 12 months only); 27.46 | CPCRA 06424 |
| Relative risk reduction on any ART regimen (%, range by CD4) | ||
| HIV-related death | 55–96 | Cotrimo-CI, ANRS 120325 |
| Acute opportunistic infections | 0–32 | Cotrimo-CI, ANRS 120325 |
| Toxicity risk (%, median time to occurrence) | ||
| Minor/moderate (all regimens) | 0.088; 3 months | ANRS 1203;48 assumption |
| Major/severe (requiring regimen change) | ||
| NVP/TDF/FTC | 12.4; 1 month | OCTANE Trial 110 |
| LPV/r/TDF/FTC | 0.8; 1 month | OCTANE Trial 110 |
| NVP/ddI/ZDV | 22.7; 3 months | Tshepo;49 assumption |
| LPV/r/ddI/ZDV | 13.2; 3 months | Tshepo;49 assumption |
|
Parameter values for “best-case” and “worst-case” scenarios for 1st-line LPV/rc | ||
| Parameter (base case value) | “Best-case” for 1st-line LPV/r | “Worst-case” for 1st-line LPV/r |
| Efficacy of NVP in 2nd-line ART (43%) | 72% (assumption, = 2nd-line LPV/r) | 16%26 |
| Efficacy of LPV/r in 2nd-line ART (72%) | 61%34 | 72% (base case) |
| Yearly risk of virologic failure after 24 weeks on LPV/r-based ART (2.84%) | 2.84% (base case) | 29.84%33 |
| Major/severe toxicity risk attributable to NVP (12.4%) | 12.4% (OCTANE) | 0.8% (Risk attributable to LPV/r in OCTANE) |
SD: standard deviation; NVP: nevirapine; LPV/r: lopinavir/ritonavir; TDF: tenofovir; FTC: emtricitabine; ZDV: zidovudine; ddI: didanosine; DRV/r: darunavir; 3TC: lamivudine, UK: United Kingdom; SAfr: South Africa; CPCRA: Community Programs for Clinical Research on AIDS; ANRS: Agence Nationale de Recherche sur le Sida.
Gain in CD4 cells at 24 weeks on suppressive ART for each OCTANE subgroup was assumed equal to that observed among the entire OCTANE cohort. CD4 gain for 2nd-line ART was assumed equal to that observed for 1st-line ART.
For OCTANE trial subgroups indicated, small numbers of events led to trial-derived risks of late virologic failure of 45.96%; late failure risks from the entire OCTANE cohort were instead included in the analyses.
These scenarios incorporated published or OCTANE-derived data most likely to bias the analysis in favor of or against the 1st-line lopinavir/ritonavir strategy.
Clinical monitoring and ART strategies in the CEPAC model
For OCTANE trial simulations, all women initiated ART upon model entry. In the base case, reflecting a combination of South African and WHO guidelines, women underwent outpatient clinical evaluation quarterly and CD4 monitoring biannually.21,22 To reflect resource availability in many settings where sdNVP is commonly used, HIV RNA monitoring was assumed to be unavailable. The first antiretroviral regimen included either lopinavir/ritonavir or nevirapine with tenofovir/emtricitabine, representing the ART regimens evaluated in the OCTANE trial. Patients were switched from the first to second antiretroviral regimen after observed clinical or immunologic failure, defined as at least one severe OI (defined in Table 1) or a ≥50% decrease from the peak on-ART CD4 count.23 The second “line” of ART included either nevirapine (following 1st-line lopinavir/ritonavir) or lopinavir/ritonavir (following 1st-line nevirapine) with didanosine/zidovudine. Reflecting practice at several OCTANE sites, women who failed either 2nd-line regimen (defined as the observation of three severe OIs or a ≥90% decrease from the peak on-ART CD4 count) received a lifelong “3rd-line/maintenance” regimen of lopinavir/ritonavir with lamivudine.21,24,25
Model input parameters (Tables 1 and 2)
Table 2.
Selected cost parameters used in a computer simulation of antiretroviral therapy strategies following exposure to single-dose nevirapine.
| Costs (2008 US dollars) | ||
|---|---|---|
| Antiretroviral therapy (monthly cost) a | Clinton Foundation12 | |
| NVP/TDF/FTC | $15.44 | Clinton Foundation12 |
| LPV/r/TDF/FTC | $50.39 | Clinton Foundation12 |
| NVP/ddI/ZDV | $23.73 | Clinton Foundation12 |
| LPV/r/ddI/ZDV | $58.68 | Clinton Foundation12 |
| “3rd-line/maintance” LPV/r/3TC | $40.96 | Clinton Foundation12 |
| 3rd line DRV/r/3TC (sensitivity analysis) | $100.93 | Médecins Sans Frontieres50 Cape Town AIDS Cohort,14 |
| Clinical care | Health Systems Trust15 | |
| Care for acute opportunistic infection (per event) | $10.71-$3330.71 | |
| Minor/moderate drug toxicity (per event) | $10.71 | |
| Major/severe drug toxicity (per event) | $1545.99 | |
| Chronic care (monthly cost) | ||
| CD4<200/μL | $51.69–129.25 | |
| CD4>200/μL | $9.84–20.77 | |
| Terminal care (last month of life) | $536.09 | |
| Laboratory monitoring (per assay) | Health Systems Trust15 | |
| CD4 test | $9.42 | |
| Viral load test (sensitivity analysis) | $47.08 | |
NVP: nevirapine; LPV/r: lopinavir/ritonavir; TDF: tenofovir; FTC: emtricitabine; ZDV: zidovudine; ddI: didanosine; DRV/r: darunavir; 3TC: lamivudine
Individual monthly drug costs: nevirapine $3.25, lopinavir/ritonavir $38.20
Baseline cohort characteristics were those of OCTANE Trial 1 participants, including mean age (31 years), CD4 (135/μL), and HIV RNA level (5.10 log10 copies/ml), and median time from sdNVP exposure (17 months).10
ART Efficacy
We defined the efficacy of each antiretroviral regimen as the 24-week rate of HIV RNA suppression to <400 copies/ml. For both 1st-line regimens, we used OCTANE Trial 1 data to derive 24-week efficacies (nevirapine: 85%, lopinavir/ritonavir: 97%), 24-week CD4 gains on each suppressive regimen, yearly risks of virologic failure >24 weeks after antiretroviral therapy initiation, and risks of severe toxicity. Second-line ART outcomes were not available from OCTANE. For 2nd-line nevirapine following 1st-line lopinavir/ritonavir, we derived the base case efficacy estimate (43%) as the midpoint of estimates from Europe (41.5%)26 and South Africa (45%).27 Data from South Africa and Malawi informed the 24-week efficacy of 2nd-line lopinavir/ritonavir following 1st-line nevirapine (72%).27,28
OCTANE subgroup analyses
To examine the impact of NNRTI resistance, the OCTANE trial also stratified participants by: 1) presence or absence of resistance detectable at ART initiation by standard genotype assays, and 2) time between sdNVP exposure and ART initiation.7 First-line ART efficacies demonstrate smaller benefits of lopinavir/ritonavir compared to nevirapine in subgroups with lower rates of known or probable NNRTI resistance (Table 1). We examined the cost-effectiveness of each 1st-line ART regimen in the OCTANE subgroups, as well as of a strategy in which 1st-line ART was selected based on detected NNRTI resistance (Appendix).
Sensitivity analyses
In sensitivity analyses, we varied clinical and cost parameters, ART switching and discontinuation strategies, and 3rd-line ART availability. We examined in detail the impact of access to HIV RNA monitoring at 3-monthly and 6-monthly intervals to facilitate earlier switching from 1st- to 2nd-line ART (Appendix). To describe the effects of simultaneous variations in multiple parameters, we performed multi-way sensitivity analyses; created “best-case” and “worst-case” clinical scenarios for 1st-line lopinavir/ritonavir, incorporating the highest and lowest published values for key model parameters (Table 1, bottom); and assigned available Trial 2 results (non-sdNVP-exposed women) to Trial 1 participants (Appendix).
Role of the funding source
The funding sources had no role in study design; collection, analysis, or interpretation of data; manuscript preparation; or decision to submit the paper for publication.
RESULTS
Model validation and short-term projections
In model validation analyses, projected clinical events at 17 months after trial enrollment (median trial follow-up duration) closely matched OCTANE observations (Table 3, top). At five years after trial enrollment, women receiving no ART were projected to experience 597 OIs/100 person-years and 1.8% survival, at a cost of $2,930/person (Table 3, bottom). In comparison, 1st-line nevirapine-based ART led to 284 projected OIs/100 person-years, 86.7% survival, and a per-person cost of $3,770. First-line lopinavir/ritonavir-based ART was associated with 257 OIs/100 person-years and 92.1% 5-year survival, at a cost of $4,680/person. Compared to 1st-line nevirapine at five years, 1st-line lopinavir/ritonavir conferred a 41% relative risk reduction in death, for an additional cost of $910 per person.
Table 3.
Short-term analyses: model validation against OCTANE trial outcomes and model-based projections for single-dose nevirapine-exposed women at 5 years after antiretroviral therapy initiation.
| A. Model validation: Outcomes at 17 monthsa | ||||
|---|---|---|---|---|
| Trial subgroups | Total OI (/person) | Survival (%) | ||
| Trial dataa | Model projections | Trial data | Model projections | |
| 1st-line NVP arm | 0.88 | 0.89 | 96.7 | 96.2 |
| 1st-line LPV/r arm | 0.84 | 0.84 | 99.2 | 97.3 |
| Entire cohort | 0.86 | 0.86 | 97.9 | 96.7 |
| B. Model results: 5-year projections | |||
|---|---|---|---|
| ART strategy | Total OIs/100 person-yearsb | Survival (%) | Mean per-person costs (2008 US$) |
| No ART | 597 | 1.8 | 2,930 |
| 1st-line NVP | 284 | 86.7 | 3,770 |
| 1st-line LPV/r | 257 | 92.1 | 4,680 |
ART: antiretroviral therapy; OI: opportunistic infection; NVP: nevirapine; LPV/r: lopinavir/ritonavir
Observed events during median duration of follow-up in OCTANE trial of 17 months. Individual person-time at risk not available for calculation of trial event rates.
Person-time at risk assumes deaths occur at mid-point of month in which they occur.
In the short term, nevirapine-based ART was projected to save money compared to no ART. For example, at two years after initiation, 1st-line nevirapine reduced projected OIs (123 versus 222 OIs/100 person-years) and improved projected survival (94.7% versus 33.6%) compared to no ART, yet was also cost-saving ($1,930 versus $2,150/person). Projected survival benefits of the 1st-line nevirapine strategy compared to no ART persisted indefinitely, and projected cost savings persisted from one to three years after ART initiation.
Long-term projections (Table 4, Section A)
Table 4.
Model-based lifetime projections for single-dose nevirapine-exposed women initiating antiretroviral therapy.
| ART strategy | Life expectancy (years) | Per-person costs (2008 US$) | Cost-effectiveness ratio ($/YLS) |
|---|---|---|---|
|
A. Entire OCTANE cohort (trial data) | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 15.2 | 13,990 | 810 |
| 1st-line LPV/r | 16.3 | 15,630 | 1,520 |
|
B. Subgroup analyses (trial data) | |||
|
Presence or absence of NNRTI resistance at ART initiation | |||
|
No baseline resistance detected | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 16.0 | 13,800 | 750 |
| 1st-line LPV/r | 16.3 | 15,600 | 5,650 |
| Baseline resistance a | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 12.2 | 14,000 | Dominated b |
| 1st-line LPV/r | 15.5 | 15,390 | 890 |
|
Time from sdNVP exposure to ART initiation | |||
| 6–12 months | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 14.2 | 14,220 | Dominated b |
| 1st-line LPV/r | 16.1 | 15,640 | 870 |
| 12–24 months | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 15.3 | 13,790 | 790 |
| 1st-line LPV/r | 16.4 | 15,650 | 1,690 |
| Greater than 24 months | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 15.2 | 13,780 | 790 |
| 1st-line LPV/r | 15.1 | 14,940 | Dominated c |
|
C. Standard genotype assay for selection of 1st-line ART | |||
| Genotype: Initiate LPV/r if NNRTI resistance detected; NVP if resistance not detected (genotype cost: $300 d) | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP for all | 15.2 | 13,990 | Dominated b |
| Genotype strategy | 15.9 | 14,320 | 790 |
| 1st-line LPV/r for all | 16.3 | 15,630 | 4,080 |
|
D. Sensitivity analyses | |||
| “Worst-case” clinical scenario for 1st-line LPV/r | |||
| No ART | 1.6 | 2,980 | |
| 1st-line LPV/r | 10.5 | 12,280 | Dominated b |
| 1st-line NVP | 13.6 | 12,370 | 780 |
| “Best-case” clinical scenario for 1st-line LPV/r | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 14.6 | 13,500 | 811 |
| 1st-line LPV/r | 17.2 | 15,670 | 813 e |
| HIV RNA monitoring available for both NVP and LPV/r strategies f | |||
| No ART | 1.6 | 2,980 | |
| 1st-line NVP | 15.3 | 15,840 | 940 |
| 1st-line LPV/r | 16.1 | 17,300 | 1,720 |
ART: antiretroviral therapy; CE: cost-effectiveness; NVP: nevirapine; LPV/r: lopinavir/ritonavir; YLS: year of life saved
Assumes 2nd-line NVP with efficacy 43% will follow 1st-line LPV/r despite detected resistance. Results change minimally (cost-effectiveness ratio for 1st-line NVP compared to no ART: $890/YLS) if NVP is avoided in 2nd-line and 1st-line LPV/r failure is followed by LPV/r/lamivudine maintenance therapy
Extended dominance: In the subgroup with baseline NNRTI resistance and the subgroup of women initiating ART within 6–12 months after sdNVP, the cost-effectiveness ratio of the 1st-line LPV/r strategy compared to 1st-line NVP is less than the cost-effectiveness ratio of 1st-line NVP compared to no ART. This indicates that the 1st-line NVP strategy represents an inefficient use of healthcare resources, if LPV/r is available. By convention in these cases, the cost-effectiveness ratio of 1st-line NVP compared to no ART is not reported. By the same mechanism, in the “worst-case” clinical scenario for 1st-line LPV/r, 1st-line LPV/r represents an inefficient use of resources compared to 1st-line NVP, and the cost-effectiveness ratio of 1st-line LPV/r compared to no ART is not shown.
Strong dominance occurs in the subgroup with sdNVP exposure >24 months prior to ART initiation because 1st-line LPV/r is both less effective and more expensive than 1st-line NVP.
Results are shown assuming a standard genotype assay cost of $300. Variations in genotype assay cost from $0-$585 do not change extended dominance of the NPV strategy by the genotype strategy. At assay costs from $585-$1000, incremental cost-effectiveness ratios of the genotype strategy compared to the nevirapine strategy range from $810–1,360/YLS. Results in table assume no HIV RNA monitoring availability; available RNA monitoring does not substantially change results.
The cost-effectiveness ratios in the “best-case” scenario are presented without rounding, to demonstrate that 1st-line nevirapine is not dominated. However, the nearly equivalent cost-effectiveness ratios of 1st-line NVP compared to no ART and of 1st-line LPV/r compared to 1st-line NVP suggest that, if all of the most favorable parameters for 1st-line LPV/r were true, any program able to afford 1st-line NVP-based ART would achieve greater health benefits at nearly the same cost by instead choosing 1st-line LPV/r.
First-line ART switched to second-line ART for a one-log increase in RNA or return to peak pre-ART RNA value. Shown are scenarios in which HIV RNA monitoring was performed every 6 months; results of 3-monthly RNA monitoring or RNA monitoring applied only to 1st-line NVP strategy are not substantially different. Lower life expectancy in the 1st-line lopinavir/ritonavir strategy with RNA monitoring, compared to the base case, result from earlier switching to a less effective 2nd-line regimen (2nd-line NVP, efficacy 43%).
Providing no ART resulted in a projected life expectancy of 1.6 years, at a cost of $2,980/person. Initiating 1st-line nevirapine-based ART increased projected life expectancy to 15.2 years and cost to $13,990/person, leading to a cost-effectiveness ratio of $810/YLS, compared to no ART. First-line lopinavir/ritonavir-based therapy increased life expectancy further, to 16.3 years, at a cost of $15,630/person. The cost-effectiveness ratio of 1st-line lopinavir/ritonavir compared to 1st-line nevirapine was $1,520/YLS.
Subgroup analyses
The cost-effectiveness ratio of 1st-line lopinavir/ritonavir compared to 1st-line nevirapine was $5,650/YLS for OCTANE participants with no detectable NNRTI resistance at baseline (Table 4, Section B). For women with baseline NNRTI resistance and for women exposed to sdNVP 6–12 months prior to ART initiation, 1st-line nevirapine represented an inefficient use of resources compared to 1st-line lopinavir/ritonavir (Table 4, footnote b). With increasing time from sdNVP exposure to ART initiation, 1st-line lopinavir/ritonavir became markedly less cost-effective, and eventually strongly dominated (>24 months). Use of 1st-line lopinavir/ritonavir only when NNRTI resistance is detected by a standard genotype assay with cost <$585 would be a more efficient use of resources than 1st-line NVP for all women (Table 4, section C).
Sensitivity analyses
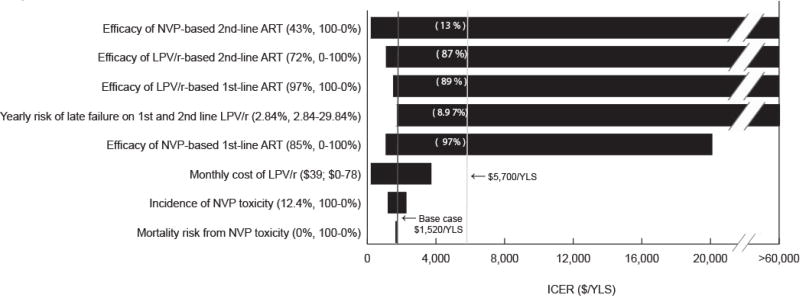
The 24-week suppressive ART efficacies and the risk of “late” virologic failure after 24 weeks on lopinavir/ritonavir-based ART had the greatest impact on the cost-effectiveness of 1st-line lopinavir/ritonavir (Figure 1). Holding all other parameters equal to the base case, 1st-line nevirapine efficacy needed to be ≤97%, 2nd-line nevirapine efficacy ≥13%, 1st-line lopinavir/ritonavir efficacy ≥89%, 2nd-line lopinavir/ritonavir efficacy ≤87%, or lopinavir/ritonavir “late” failure risk ≤8.97%/year (1.64%/year lower than nevirapine “late” failure), for the cost-effectiveness ratio of 1st-line lopinavir/ritonavir to remain <$5,700/YLS (South African GDP).
Figure 1.
NVP: nevirapine; ART: antiretroviral therapy; LPV/r: lopinavir/ritonavir; ICER: incremental cost-effectiveness ratio; YLS: year of life saved
A “tornado diagram” summarizes the results of a series of 1-way sensitivity analyses. Key model parameters are listed on the vertical axis. For each parameter, the value used in the base case analysis and the range examined in sensitivity analyses are listed in parentheses. The horizontal axis represents cost-effectiveness ratios for 1st-line LPV/r compared to 1st-line NVP. Each horizontal bar represents the range of cost-effectiveness ratios produced by varying model parameter across the ranges shown. The solid vertical line denotes the base case cost-effectiveness ratio estimate ($1,520/YLS, and the dashed vertical line denotes the cost-effectiveness ratio of $5,700 (2008 South African GDP) per YLS. Numbers in parentheses above each horizontal bar indicate the threshold value of each parameter at which the cost-effectiveness ratio for 1st-line LPV/r compared to nevirapine becomes <$5,700/YLS. The horizontal axis should be understood to extend to the right beyond $60,000/YLS and to include instances when 1st-line LPV/r costs more and dominates 1st-line NVP (as is the case when 2nd-line LPV/r efficacy is ≥90%). Bars abutting the far left margin indicate situations in which 1st-line LPV/r costs less and confers more life-years than 1st-line NVP, or in which 1st-line NVP is dominated (see Table 4, footnote b).
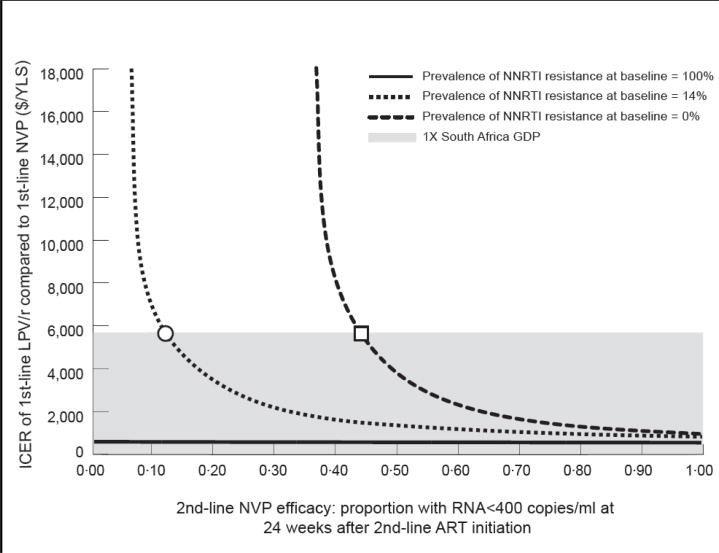
The cost-effectiveness ratio for 1st-line lopinavir/ritonavir remained <$5,700/YLS even if the cost of lopinavir/ritonavir was more than twice its current value (Figure 1), as well as if HIV RNA monitoring was available (Table 4). Results were robust to wide variations in CD4 counts at sdNVP receipt and ART initiation; incidence and severity of nevirapine-related toxicity; ART monitoring, switching, and stopping strategies; and composition and availability of 3rd-line ART (Supplementary Table 2). The influence of 2nd-line ART efficacy on cost-effectiveness results was greatest when the prevalence of baseline NNRTI resistance was very low (Figure 2).
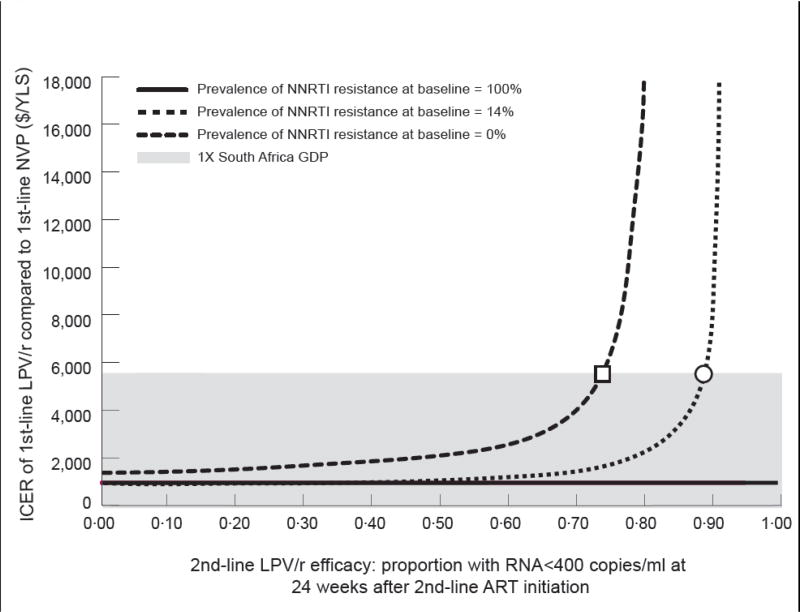
Figure 2.
ICER: incremental cost-effectiveness ratio; LPV/r: lopinavir/ritonavir; NVP: nevirapine; YLS: year of life saved; GDP: gross domestic product; NNRTI: non-nucleoside reverse transcriptase inhibitor; ART: antiretroviral therapy.
Two-way sensitivity analyses depicting the simultaneous impact of the prevalence of NNRTI resistance at baseline and the efficacy of the 2nd-line ART in suppressing HIV RNA to <400 copies/ml at 24 weeks. In both panels, the vertical axis indicates the cost-effectiveness ratio for 1st-line LPV/r compared to 1st-line NVP in $/YLS. The vertical axis should be understood to extend beyond the $18,000/YLS mark, to include situations in which 1st-line LPV/r is “dominated” by 1st-line NVP. The shaded regions indicate a cost-effectiveness ratio of <$5,700/YLS.
In panel A, the horizontal axis represents varying values for 2nd-line NVP efficacy. The green line indicates the OCTANE cohort in whom no NNRTI resistance was detected by standard genotypic resistance testing at the time of ART initiation. In this group, 2nd-line NVP efficacy must be ≥44% (square) for the 1st-line LPV/r cost-effectiveness ratio to fall below $5,700. The blue line indicates the base case scenario for the entire OCTANE cohort, in whom the prevalence of NNRTI resistance at baseline was 14%. For this group, 2nd-line NVP efficacy must be ≥13% (circle) for the 1st-line LPV/r cost-effectiveness ratio to fall below $5,700. The purple line indicates the OCTANE cohort in whom NNRTI resistance was detected at baseline. For this group, 1st-line LPV/r is economically preferred to 1st-line NVP at all values of 2nd-line NVP efficacy, due to “extended dominance” (purple line). The cost-effectiveness ratios of LPV/r compared to no antiretroviral therapy are displayed in the Figure.
In panel B, the horizontal axis represents values for 2nd-line LPV/r efficacy. In the OCTANE cohort in whom no baseline NNRTI resistance was detected (green line) 2nd-line LPV/r efficacy must be ≤73% (square) for the 1st-line LPV/r cost-effectiveness ratio to fall below $5,700. In the entire OCTANE cohort (14% baseline resistance, blue line), 2nd-line LPV/r efficacy must be ≤87% (circle) for the 1st-line LPV/r cost-effectiveness ratio to fall below $5,700. For the group in whom NNRTI resistance was detected at baseline (purple line), 1st-line LPV/r is again economically preferred to 1st-line NVP at all values of 2nd-line LPV/r efficacy, due to “extended dominance” (purple line). The cost-effectiveness ratio of LPV/r compared to no ART are displayed in the Figure.
Additional multi-way sensitivity analyses indicated that using “worst-case” parameter values for the 1st-line lopinavir/ritonavir strategy led 1st-line lopinavir/ritonavir to be dominated by 1st-line nevirapine (Table 4, Section C). “Best-case” values for 1st-line lopinavir/ritonavir rendered the cost-effectiveness ratio of lopinavir/ritonavir compared to nevirapine similar to that of nevirapine compared to no antiretroviral therapy ($810/YLS). When both lopinavir/ritonavir 1st-line efficacy (93%) and “late” failure risk (8.08%/year) from Trial 2 were applied to Trial 1 participants (but not when each parameter was applied alone), lopinavir/ritonavir cost-effectiveness was substantially reduced (Appendix).
DISCUSSION
We report model-based projections of clinical outcomes and costs for South African women, similar to OCTANE Trial 1 participants, initiating ART a median of 17 months after sdNVP exposure for prevention of mother-to-child transmission. From one to three years after ART initiation, nevirapine-based 1st-line ART is projected to improve survival and save money compared to the use of no ART. Short-term cost savings stem from the marked reduction in OIs and death associated with nevirapine-based ART compared to no ART; costs averted in OI and end-of-life care offset the costs of nevirapine-based ART itself. By reducing risk for early virologic failure, use of 1st-line lopinavir/ritonavir instead of 1st-line nevirapine adds modest short-term benefit and cost, improving 5-year survival from 86.7% to 92.1% at an additional cost of $910/person.
In projections beyond 3 years, providing nevirapine-based ART becomes more expensive than providing no ART, due to the prolonged medication and care costs accrued during markedly longer life expectancies (15.2 vs. 1.6 years). By WHO guidance, this substantial survival benefit from nevirapine-based ART would be “very cost-effective” compared to no ART (cost-effectiveness ratio: $810/YLS). First-line lopinavir/ritonavir further increases life expectancy to 16.3 years, with a higher cost-effectiveness ratio ($1,520/YLS) compared to 1st-line nevirapine. This suggests that for HIV programs able to afford higher per-person costs for HIV care ($4,680 vs. $ 3,770 at 5 years; $15,630 vs. $13,990 over patient lifetimes), 1st-line lopinavir/ritonavir provides excellent value for sdNVP-exposed women.
These findings confirm modeling work conducted before OCTANE data were available29 and are robust to plausible variations in HIV RNA monitoring availability and frequency, lopinavir/ritonavir cost, nevirapine toxicity, CD4 counts, and availability of 3rd-line ART. However, three factors influence the cost-effectiveness of 1st-line lopinavir/ritonavir: 1st-line lopinavir/ritonavir efficacy, long-term ART outcomes, and NNRTI resistance at ART initiation.
First-line lopinavir/ritonavir remains “very cost-effective” compared to nevirapine unless its 24-week suppressive “efficacy” is <89% (holding 1st-line nevirapine efficacy at 85%). While OCTANE Trial 1 demonstrated a 24-week efficacy far above this threshold (97%), other trials have suggested 24-week intention-to-treat lopinavir/ritonavir efficacies ranging from 65–93% in treatment-naïve women without sdNVP exposure.30,31 If future studies show the efficacy of 1st-line lopinavir/ritonavir efficacy among sdNVP-exposed women to be at the lower end of this range, lopinavir/ritonavir cost-effectiveness will be lower than Trial 1 data suggest.
The cost-effectiveness of lopinavir/ritonavir is also influenced by two long-term ART outcomes: the efficacy of 2nd-line ART and the risk of “late” virologic failure after 24 weeks on ART. A primary concern about the use of first-line lopinavir/ritonavir in women with sdNVP exposure is the feasibility of “returning” to nevirapine-based 2nd-line ART. Because 2nd-line antiretroviral therapy in many settings is often protease inhibitor-based,21 the 24-week suppressive efficacy of 2nd-line NNRTI-based antiretroviral therapy is infrequently described. Available estimates are from observational studies in the United Kingdom26 and Durban, South Africa27 (Appendix), and range from 16.0–45.0%. Theoretically, the lowest published 2nd-line nevirapine efficacies may be applicable to OCTANE participants. If women randomized to nevirapine failed 1st-line antiretroviral therapy due to sdNVP-associated resistance, but those randomized to lopinavir/ritonavir failed 1st-line antiretroviral therapy due to poor adherence, this poor 1st-line adherence may predict poor adherence to, and low efficacy of, the 2nd-line nevirapine-based regimen that follows 1st-line nevirapine.27 Alternatively, the true efficacy of 2nd-line nevirapine for OCTANE participants may be higher than the published values, because OCTANE participants initiated completely novel 2nd-line regimens, including two NRTIs to which they had no previous exposure. In addition, greater time since sdNVP exposure in those initiating 1st-line lopinavir/ritonavir (due to time elapsed before initiating 2nd-line nevirapine) might improve 2nd-line nevirapine efficacy due to attenuation of single-dose nevirapine-associated NNRTI resistance with time.5,32 Although the base case estimate (43%) was chosen to be conservative with regard to these assumptions, we find that first-line lopinavir/ritonavir remains “very cost-effective” unless the 24-week efficacy of the subsequent 2nd-line nevirapine-based regimen is <13%, lower than the published estimates of 16–45.26,27 Similarly, the efficacy of 2nd-line lopinavir/ritonavir (following 1st-line nevirapine) must exceed 87% for the 1st-line lopinavir/ritonavir strategy to no longer be “very cost-effective,” also outside published ranges (45–75%28,33–36).
In addition, Trial 1 data demonstrated substantially greater “late” failure risks for NVP (10.61%/year) than lopinavir/ritonavir (2.84%/year). Sensitivity analyses (Figure 1) highlight that if the risk of “late” failure on lopinavir/ritonavir approached or exceeded that of nevirapine, lopinavir/ritonavir would be less cost-effective. However, when data from other published trials are used to determine “late” failure risks, reported ranges for NNRTI- (<0–17.60%/year) and protease-inhibitor- (<0–29.84%/year) based ART are wide and overlapping.26,28–30,35–38 As interest in protease inhibitor-based 1st-line ART increases for men and for women without sdNVP exposure in resource-limited settings,30,39 data on late outcomes of 1st-line ART and virologic suppression on 2nd-line ART should remain a research priority.
The impact of NNRTI resistance is reflected in the OCTANE subgroups stratified by 1) NNRTI resistance at baseline, and 2) time between sdNVP exposure and ART initiation. Comparing the primary trial endpoint (virologic failure or death), lopinavir/ritonavir was markedly superior to nevirapine among the entire Trial 1 cohort and among women with detectable NNRTI resistance at ART initiation. However, among participants without detectable NNRTI resistance, lopinavir/ritonavir conferred a smaller, non-significant benefit over nevirapine.10 As a result of this trial-reported decrease in relative clinical effectiveness, we find that 1st-line lopinavir/ritonavir becomes less cost-effective as the population prevalence of NNRTI resistance decreases. Additionally, as time between sdNVP exposure and ART initiation increases, sdNVP-associated resistance fades from detection,32 and the efficacy of NNRTI-based ART improves.5,8,32,40,41 Because the superiority of lopinavir/ritonavir compared to nevirapine similarly faded with time in OCTANE, we find that 1st-line lopinavir/ritonavir is most cost-effective for women with recent sdNVP exposure, “dominating” 1st-line nevirapine if ART is initiated within 6–12 months after sdNVP.
This analysis suggest that genotypic resistance testing at ART initiation is a cost-effective strategy for selection of 1st-line ART after sdNVP exposure. However, if resistance testing or 1st-line lopinavir/ritonavir is not readily available, efforts to reduce sdNVP-associated resistance remain critical. This analysis focuses on women who have already been exposed to sdNVP and who will require ART in the near future.2,5,6 Expansion of more effective, non-sdNVP-based antiretroviral regimens for the prevention of mother-to-child transmission11,42 will eventually lead to sdNVP exposure in fewer women. Similarly, improved access to HIV diagnosis early in pregnancy will reduce the number of women who present for PMTCT services in labor and thus require sdNVP.43 When available, the recommended administration of dual-NRTI “tails” following sdNVP may further reduce sdNVP-associated resistance by up to 80%.11,42,44 Finally, more widespread access to ART for women with advanced HIV disease, as currently recommended by the WHO11 and the South African National Department of Health,3 will further reduce the number of women exposed to sdNVP. Each of these interventions will thereby help to preserve the effectiveness of inexpensive, NNRTI-based ART for HIV-infected women after delivery.40,45
There are four primary limitations to this analysis. First, although clinical and cost data from South Africa may not be generalizable to all settings where sdNVP is used, we tested a wide range of ART availability, monitoring strategies, and drug costs. These had no substantial impact on policy conclusions unless the most unfavorable values for 1st-line lopinavir/ritonavir were incorporated simultaneously. Second, interventions deemed “very cost-effective” by WHO-recommended, GDP-based guidelines in South Africa, a middle-income country, may not be considered so in many other countries where sdNVP continues to be widely used.18,46 Third, application of Trial 2 results (non-sdNVP-exposed women)30 to the Trial 1 cohort reduces lopinavir/ritonavir cost-effectiveness. However, Trial 2 results may not be extrapolable to sdNVP-exposed women, due to notable differences in the Trial 1 and 2 cohorts (Appendix), and Trial 2 lopinavir/ritonavir 24-week efficacy and “late” failure must be applied simultaneously to impact the cost-effectiveness of lopinavir/ritonavir compared to nevirapine. Finally, by convention, cost-effectiveness analyses assume a lifetime perspective.17 Model parameters reflecting current practice, including availability of 2nd- and 3rd-line ART and prevalence of sdNVP-associated resistance, may be inappropriate for the distant future. We therefore projected 2–5-year clinical outcomes and costs. These validated short-term estimates may inform the design of clinical trials and may assist national HIV programs and funders planning near-term HIV-related budgets.
CONCLUSIONS
Among sdNVP-exposed South African women similar to OCTANE participants, initiating nevirapine-based ART is cost-saving in the short-term, and very cost-effective in the long-term, compared to no ART. Initiating lopinavir/ritonavir-based ART improves survival further and is very cost-effective in South Africa, compared to initiating nevirapine-based ART. Based on OCTANE Trial 1 data, initial lopinavir/ritonavir-based ART should be recommended for women exposed to sdNVP <12 months prior to ART initiation and for women in whom a standard genotype assay detects NNRTI-resistant HIV. Where resources permit, lopinavir/ritonavir should also be recommended for women exposed 12–24 months prior and for sdNVP-exposed women for whom timing and resistance information is unknown.
Supplementary Material
Acknowledgments
Funding sources: Funding for this work was provided by the National Institute of Allergy and Infectious Disease (K01 AI078754 (ALC), K24 AI56933 (J.Currier), K24 AI062476 (KAF), R01 AI058736 (KAF, RPW, ALC, RW, J.Chu, EL), R01 HD044391 (SL), P30 AI 60354 (ALC), R01 AI 69453 (JM)); the Adult AIDS Clinical Trials Group (U01 AI068636 (ALC, KAF, RPW), U01 AI069424 (J.Currier), Statistical and Data Management Center for the AIDS Clinical Trials Group (U01 AI68634 (MDH)); the Comprehensive International Program for Research on AIDS in South Africa (U19 AI53217 (RW)); and the Elizabeth Glaser Pediatric AIDS Foundation (ALC, KAF, J.Chu, RPW).
The authors gratefully acknowledge Evelyn Zheng for detailed analysis of OCTANE trial data; Paul Sax, Martin Hirsch, and Rajesh Gandhi for interpretation of data regarding 2nd-line NNRTI efficacy; Richard Murphy, Vincent Marconi, Henry Sunpath, Zhigang Lu, and Daniel Kuritzkes for assistance in incorporating 2nd-line antiretroviral therapy data from Durban, South Africa; and Asinath Rusibamayila and Ji-Eun Park for assistance in manuscript preparation.
We are indebted to the entire CEPAC-International team and investigators for their contributions, including Christine Danel, Thérèse N’Dri-Yoman, Eugène Messou, Raoul Moh, Eric Ouattara, Catherine Seyler, and Siaka Touré (Programme PACCI, Abidjan, Côte d’Ivoire); Yazdan Yazdanpanah (Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, EA 2694, Faculté de Médecine de Lille, and Laboratoire de Recherches Économiques et Sociales, Centre National de la Recherche Scientifique Unité de Recherche Associée 362, Lille, France); Xavier Anglaret, Delphine Gabillard, Hapsatou Touré (INSERM U897, Université Bordeaux 2, Bordeaux, France); Nagalingeswaran Kumarasamy and A. K. Ganesh (Y.R. Gaitonde Centre for AIDS Research & Education, Chennai, India); Catherine Orrell and Robin Wood (University of Cape Town, Cape Town, South Africa); Neil Martinson and Lerato Mohapi (Perinatal HIV Research Unit, WITS Health Consortium, Johannesburg, South Africa); Kara Cotich, Sue J. Goldie, April D. Kimmel, Marc Lipsitch, Alethea McCormick, Chara Rydzak, and George R. Seage III, and Milton C. Weinstein (Harvard School of Public Health, Boston, MA, USA); C. Robert Horsburgh (Boston University School of Public Health); Heather E. Hsu (Harvard Medical School, Boston, MA, USA); Timothy Flanigan and Kenneth Mayer (Miriam Hospital, Providence, RI, USA); A. David Paltiel (Yale University, New Haven, CT, USA); Aima Ahonkhai, Jason Andrews, Ingrid V. Bassett, Jessica Becker, Melissa A. Bender, John Chiosi, Julie Levison, Benjamin P. Linas, Zhigang Lu, Sarah Lorenzana, Bethany Morris, Mai Pho, Erin Rhode, Callie A. Scott, Caroline Sloan, Adam Stoler, Lauren Uhler, Bingxia Wang, and Angela Wong (Massachusetts General Hospital, Boston, MA, USA).
We are also indebted to the CEPAC-International Scientific Advisory Board, including Richard Chaisson, Victor De Gruttola, Joseph Eron, R.R. Gangakhedkar, Jonathan Kaplan, Salim Karim, Thérèse N’Dri Yoman, Douglas Owens, and John Wong.
The authors also gratefully acknowledge the efforts of the OCTANE trial investigator team, including Tsungai Chipato and James Hakim- (UZ-Parirenyatwa CRS (Site 30313),CTU Grant #U01AI069436); Mohammed S Rassool and Josephine Tsotsotetsi (WITS HIV Research Group (Site 11101), CTU Grant #U01 AI69463-03); Fred Sawe and Douglas Shaffer KMRI/Walter Reed Project Clinical Research Center (Site 12501); Charity Potani and Regina Mwausegha (UNC Project, Kamuzu Central Hospital (Site 12001), CTU Grant #5 U01 AI069518; Fatima Laher and Reinet Hen-Boisen (Soweto ACTG CRS (Site 12301), CTU Grant # AI69453); Kipruto Kirwa and Agnes Nzioka- Moi University CRS (Site 12601), CONTRACT No. AACTG. 50.5208.07, the United States Military HIV Research Program); Margaret Chibowa and Elizabeth Stringer (Centre for Infectious Disease Research, Kalingalinga (Site 12801), CTU Grant #5U01AI069455-03 and # 3U01AI32775-13S5); Kagiso Sebina, Kinuthia Mburu, and Tebogo Kakhu (Botswana Harvard Project, Gaborone-Princess Marina Hospital (Site 12701), CTU Grant #5U01AI069456-03); Diana Atwine, Cissy Kityo, Peter Mugyenyi, and Sandra Rwambuya (Site 12401), CTU Grant #AI-069501; Site 11201; and Banno Moorad (Botswana Harvard Project, Molepolole Unit (Site 12702)).
Footnotes
Dr. Ciaranello had full access to all data reported in this manuscript and assumes responsibility for the final decision to submit this manuscript for publication.
This work was presented in part at the 2009 Meeting of the International AIDS Society (Cape Town, South Africa, July 2009).
All authors contributed substantively to this manuscript in the following ways: Study design (ALC, SL, KAF, MH, JChu, CBH, RPW), data collection and analysis for derivation of model input parameters (ALC, SL, KAF, MH, JCurrier, RW, SP, FC, JM, EL, RPW), model development (KAF, EL, RPW), execution of model analyses (ALC, JChu), interpretation of model results (ALC, SL, KAF, MH, JChu, EL, RPW), drafting the manuscript (ALC), and critical revision of the manuscript (all authors).
The OCTANE trial is registered on ClinicalTrials.gov (NCT 00089505) and supported by NIAID (U01 AI06836, AI68634) and the General Clinical Research Centers (GCRC) of the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID or the National Institutes of Health.
Conflicts of interest: Michael Hughes is a paid member of Data and Safety Monitoring Boards for the following manufacturers of antiretroviral therapy: Boehringer Ingelheim, Pfizer, Tibotec. James McIntyre has received speaker’s honoraria from Abbott Pharmaceuticals, and research funding, travel grants and speaker’s honoraria from Boehringer Ingelheim and Glaxo SmithKline. All other authors have no conflicts of interest to disclose.
References
- 1.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. [Accessed July 1, 2010];Progress Report. 2009 http://www.who.int/hiv/pub/tuapr_2009_en.pdf.
- 3.South Africa National Department of Health. Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) [Accessed October 27, 2010];2010 http://www.doh.gov.za/docs/factsheets/guidelines/pmtct.pdf.
- 4.World Health Organization. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. [Accessed October 27, 2010];Progress Report. 2010 http://www.who.int/hiv/pub/2010progressreport/report/en/index.html.
- 5.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 6.South Africa National Department of Health. [Accessed October 27 2010];Policy and Guidelines for the Implementation of the PMTCT Programme. 2008 http://www.doh.gov.za/docs/policy/pmtct.pdf.
- 7.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–21. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 8.Stringer JS, McConnell MS, Kiarie J, et al. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med. 7:e1000233. doi: 10.1371/journal.pmed.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–40. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 10.Lockman S A5208/OCTANE Study Team. Abstract 94LB: Lopinavir/ritonavir+tenofovir/emtricitabine is superior to nevirapine+tenofovir/emtricitabine for women with prior exposure to single-dose nevirapine: A5208 (“OCTANE”). Conference on Retroviruses and Opportunistic Infections; 2009; Montreal, Canada. 2009. [Google Scholar]
- 11.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. [Accessed October 28, 2010];2010 http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
- 12.William J Clinton Foundation. [Accessed November 2, 2010];Clinton Foundation HIV/AIDS Initiative (CHAI) Antiretroviral Price List. 2009 August; http://www.clintonfoundation.org/files/chaiarvpricelistaugust2009english.pdf.
- 13.Walensky RP, Wolf LL, Wood R, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–66. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 15.Cleary SBA, McIntyre D, Coetzee D. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. [Accessed November 2, 2010];2004 http://www.hst.org.za/uploads/files/arv_cost.pdf.
- 16.World Bank. [Accessed October 28, 2010];World Development Indicators. 2010 http://data.worldbank.org/data-catalog/world-development-indicators.
- 17.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 18.World Health Organization (WHO-CHOICE) Prices for hospitals and health centers. World Health Organization; Geneva: 2010. [Accessed October 27, 2010]. http://www.who.int/choice/en/ [Google Scholar]
- 19.International Monetary Fund. World Economic and Financial Surveys: World Economic Outlook Database WEO Groups and Aggregates Information. [Accessed October 28, 2010];2008 http://www.imf.org/external/pubs/ft/weo/2008/01/weodata/groups.htm#oem.
- 20.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Côte d’Ivoire. N Engl J Med. 2006;355:1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. [Accessed October 27, 2010];2006 http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 22.South Africa National Department of Health. [Accessed October 27, 2010];Antiretroviral Treatment Guidelines. 2010 http://www.doh.gov.za/docs/factsheets/guidelines/art.pdf.
- 23.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents - Recommendations for a public health approach (2010 version) [Accessed October 27, 2010];2010 http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 24.Lawrence J, Mayers DL, Hullsiek KH, et al. Structured treatment interruption in patients with multidrug-resistant human immunodeficiency virus. N Engl J Med. 2003;349:837–46. doi: 10.1056/NEJMoa035103. [DOI] [PubMed] [Google Scholar]
- 25.Losina E, Yazdanpanah Y, Deuffic-Burban S, et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Côte d’Ivoire. Antivir Ther. 2007;12:543–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Asboe D, Mandalia S, Gazzard BG. Sequencing to NRTI plus NNRTI-only combinations after virological failure of protease inhibitor-based combination HIV-1 therapy. HIV Clin Trials. 2003;4:1–10. doi: 10.1310/P19C-CCEK-R62L-1G91. [DOI] [PubMed] [Google Scholar]
- 27.Murphy R, Lu Z, Sunpath S, et al. Abstract 658: Response to second-line ART in South Africa after antiretroviral drug-resistance testing. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 28.Hosseinipour MKJ, Weigel R, Brown L, Mzinganjira D, Mhango B, Phiri S, Van Oosterhout J. Abstract # 605: Clinical, immunological, and virological outcomes of second-line treatment in Malawi. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 29.Holmes CB, Zheng H, Martinson NA, Freedberg KA, Walensky RP. Optimizing treatment for HIV-infected South African women exposed to single-dose nevirapine: balancing efficacy and cost. Clin Infect Dis. 2006;42:1772–80. doi: 10.1086/504382. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre JHM, Mellors J, Zheng Y, Hakim J, Asmelash A, Conradie F, Schooley R, Currier J, Lockman S. Efficacy of antiretroviral treatment (ART) with nevirapine (NVP) + tenofovir/emtricitabine (TDF/FTC) vs. lopinavir/ritonavir (LPV/r) + TDF/FTC among antiretroviral-naïve women in Africa: OCTANE Trial 2/ACTG A5208. Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- 31.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 32.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–78. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 34.Johnson MGB, Rodriguez C. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 35.Fox MIP, Malope-Kgokong B, Long L, Sanne I. Abstract #606: Clinical Outcomes on Second-line ART in a Large Urban Clinic in Johannesburg, South Africa. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 36.Castelnuovo B, John L, Lutwama F, et al. Three-year outcome data of second-line antiretroviral therapy in Ugandan adults: good virological response but high rate of toxicity. J Int Assoc Physicians AIDS Care (Chic Ill) 2009;8:52–9. doi: 10.1177/1545109708328538. [DOI] [PubMed] [Google Scholar]
- 37.Coetzee D, Hildrebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 38.Delfraissy JF, Flandre P, Delaugerre C, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. AIDS. 2008;22:385–93. doi: 10.1097/QAD.0b013e3282f3f16d. [DOI] [PubMed] [Google Scholar]
- 39.Adlington R, Richens J, Shahmanesh M. First-line antiretroviral therapy in resource-limited settings: time to reconsider? J Infect Dis. 2009;199:1407. doi: 10.1086/597809. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn L, Semrau K, Ramachandran S, et al. Mortality and virologic outcomes after access to antiretroviral therapy among a cohort of HIV-infected women who received single-dose nevirapine in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181ab6d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coovadia A, Hunt G, Abrams EJ, et al. Persistent Minority K103N Mutations among Women Exposed to Single-Dose Nevirapine and Virologic Response to Nonnucleoside Reverse-Transcriptase Inhibitor-Based Therapy. Clin Infect Dis. 2009 doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Department of Health and South African National AIDS Council. Clinical guidelines: PMTCT (Prevention of mother-to-child transmission) National Department of Health; South Africa: 2010. [Google Scholar]
- 43.UNAIDS/UNICEF/WHO. [Accessed October 27, 2010];Children and AIDS: Fourth stocktaking report, actions and progress. 2009 http://www.unicef.org/publications/index_46585.html.
- 44.McIntyre JA, Hopley M, Moodley D, et al. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial. PLoS Med. 2009;6:e1000172. doi: 10.1371/journal.pmed.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arrive E, Chaix ML, Nerrienet E, et al. Tolerance and viral resistance after single-dose nevirapine with tenofovir and emtricitabine to prevent vertical transmission of HIV-1. AIDS. 2009;27:825–33. doi: 10.1097/QAD.0b013e32832949d5. [DOI] [PubMed] [Google Scholar]
- 46.World Bank. [Accessed October 27 2010];Data and statistics: Country classification. 2009 http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,,contentMDK:20420458~menuPK:64133156~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html.
- 47.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 48.Seyler C, Anglaret X, Dakoury-Dogbo N, et al. Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Côte d’Ivoire. Antivir Ther. 2003;8:385–93. [PubMed] [Google Scholar]
- 49.Bussmann H, Wester CW, Thomas A, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C-infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009;51:37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Médecins Sans Frontières. Untangling the web of price reductions: A pricing guide for the purchase of ARV’s in developing countries. [Accessed November 2, 2010];2008 http://www.msf.org.za/Docs/untangling_the_web.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.