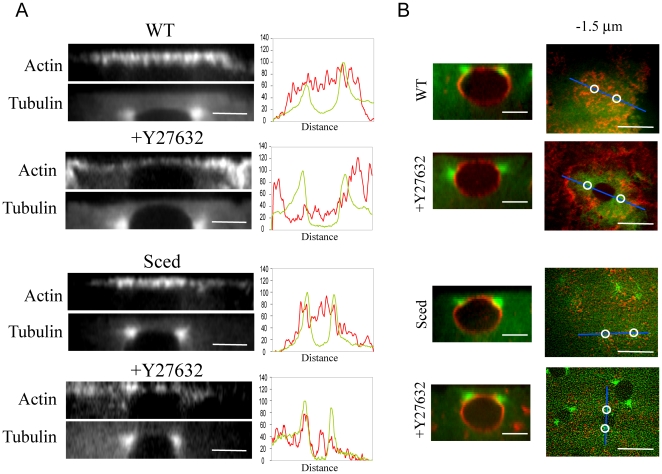
Figure 6. Effect of myosin inhibition on actin distribution in WT and Sced embryos.
A) Side views of actin at the cortex in WT and Sced embryos in the absence and presence of the myosin inhibitor Y27632 reveal different actin distributions and cap sizes. The WT actin cap is compact with an evenly distributed thick layer of actin. When myosin is inhibited, the cap expands remaining thick at the margins and becoming thinner in the center. Note that the nucleus ascends ‘indenting’ the cap. In Sced embryos the cap becomes smaller compared to WT embryos, and when myosin function is inhibited, the actin in the cap becomes disorganized. Corresponding line scans of fluorescence intensity (tubulin, green; actin, red) taken along the actin cap in relation to the centrosomes' position (taken along the pole-pole axis), confirm the qualitative description of the changes in actin distribution. Bars, 5 µm. B) Side view of the centrosomes' position with respect to the nucleus. The nuclear membrane was stained in vivo with fluorescent conjugated WGA in WT embryos expressing GFP-tubulin or in Sced embryos injected with FITC-tubulin. When myosin is inhibited in WT, the centrosomes move further down and around the nucleus than in control. In Sced, the centrosomes are positioned slightly more apically, and when myosin in inhibited, this apical shift and lesser separation becomes more evident. Bars, 10 µm. Next to the side views are corresponding images of a wild-type embryo expressing GFP-tubulin (green) injected with rhodamine-actin (red) and a Sced embryo injected with FITC-tub (green) and rhodamine-actin (red) in the presence and absence of the myosin inhibitor Y27632. The prophase actin cap taken 1.5 µm below the top shows the difference in actin density and distribution between wild-type and Sced embryos in the presence or absence of myosin. Circles mark the position of the centrosomes which in some cases are not clearly visible. Bars, 10 µm.