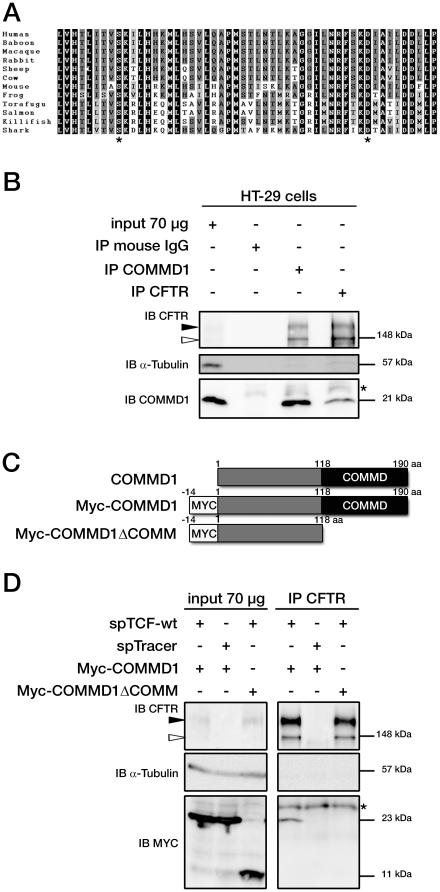
Figure 1. COMMD1 and CFTR interact in mammalian cells.
(A) Sequences of ICL3 in other species from fish to primates. Asterisks indicate the position of two class II mutations: S945L and D979A. Identity of amino acids between the different proteins are boxed in black, conserved residues are boxed in dark gray and semi-conserved substitutions in light gray. (B) Representative gels for the same co-immunoprecipitation experiments in HT-29 cells expressing endogenous CFTR and COMMD1. Lysates from HT-29 cells were immunoprecipitated (IP) with either 0.8 µg of anti-COMMD1 mAb (Abnova), 0.8 µg of anti-CFTR mAb (MAB25031, R&D Systems) or with 0.8 µg anti-mouse IgG as a control. Each immunoprecipitation sample was then split in half and loaded onto an 8% SDS-PAGE for CFTR detection and 11% SDS-PAGE for COMMD1 detection. Both gels were transferred to PVDF membrane and subjected to immunoblotting (IB). The 8% SDS-PAGE membrane was probed with anti-CFTR mAb (MM13-4) and the 11% SDS-PAGE membrane with a rabbit anti-COMMD1 pAb (Proteintech Group). Both membranes were probed with anti-α-tubulin as control (11% gel is shown). Filled and empty arrowheads indicate the fully- (170 kDa) and core-glycosylated (140 kDa) CFTR, respectively. * indicates mouse IgG light chain from the antibody used for immunoprecipitation. (C) COMMD1 constructions in pcDNA3.1/Topo plasmid. Two COMMD1 constructs were generated by adding a Myc-tag at the N-terminus of COMMD1: Myc-COMMD1 and a construct with a deletion of the COMM domain named Myc-COMMD1ΔCOMM. (D) Representative gels for the same co-immunoprecipitation experiment between COMMD1 and wt- in heterologous system. HeLa cells stably expressing wt- (spTCF-wt) or empty CFTR vector (spTracer) as control were transfected with Myc-COMMD1. spTCF-wt were transfected with Myc-COMMD1ΔCOMM. Lysates from all these experiments were subjected to SDS-PAGE, as in (B) after CFTR IP. The 8% SDS-PAGE membrane was probed with anti-CFTR mAb and the 11% SDS-PAGE membrane with anti-c-Myc mAb. Both membranes were probed with anti-α-tubulin as control (11% gel is shown).