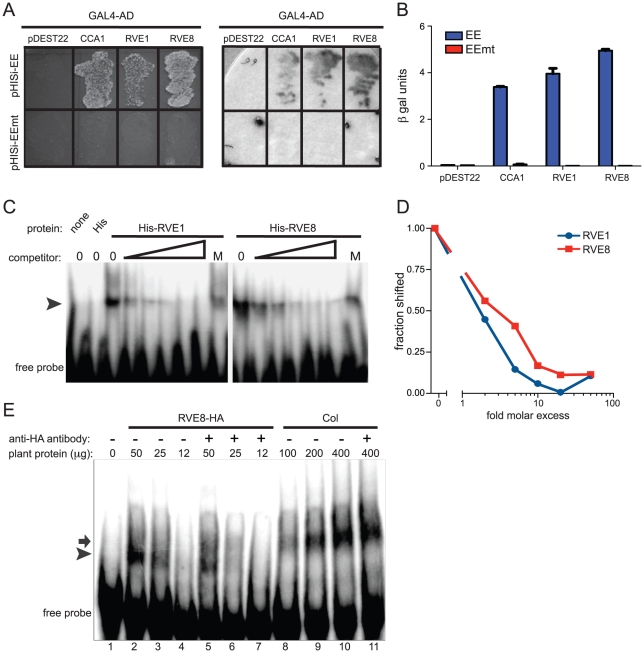
Figure 2. RVE8 binds specifically to the EE both in vivo and in vitro.
(A, B) Four copies of wild-type or mutant EE were multimerized and used to drive expression of the HIS3 or lacZ genes in S. cerevisiae. The CCA1, RVE1, and RVE8 cDNAs were fused to the GAL4 activation domain, transformed into yeast containing the above bait vectors, and plated on media lacking histidine (A, left panel). They were also assayed for β-galactosidase activity on a filter (A, right panel) and in a liquid assay (B). (C) Recombinant RVE1 or RVE8 was incubated with a multimerized, radiolabeled, EE probe sequence. A 2, 5, 10, 20, or 50-fold molar excess of unlabeled EE or a 50-fold molar excess of unlabeled mutant EE (indicated by the letter M) was added to each reaction as competitor. Protein/DNA complexes were separated on a non-denaturing polyacrylamide gel and the radiolabeled DNA visualized using a phosphorimager. The arrowhead indicates the position of protein/DNA complexes. (D) The fraction of probe shifted in each lane was quantified and normalized to the fraction shifted in the lane with no added competitor. (E) An EMSA assay was performed with protein extracted from wild-type or plants overexpressing HA-tagged RVE8. 1 µg of anti-HA antibody was added to some reactions, as indicated. The arrow indicates the mobility of the shifted DNA/protein complex using extracts made from wild type while the arrowhead indicates the mobility of the complex using extracts made from plants overexpressing RVE8. Data are representative of three independent experiments.