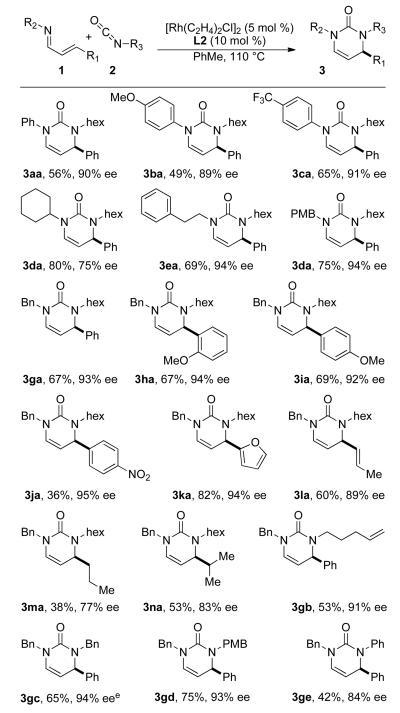
Chart 1.
Reaction scope.a
a Conditions: 1 (0.3 mmol), 2 (1.25 equiv), [Rh(C2H4)2Cl]2 (5 mol %), and L2 (10 mol %) in PhMe at 110 °C for 12 h. b Isolated yield. c Enantiomeric excess determined by HPLC using a chiral stationary phase. d Absolute configuration assigned by analogy to (R)-3af (established by X-ray analysis – see SI). e On 4.5 mmol scale with [Rh(C2H4)2Cl]2 (1 mol %) and L2 (2 mol %): 71% yield and 94% ee.