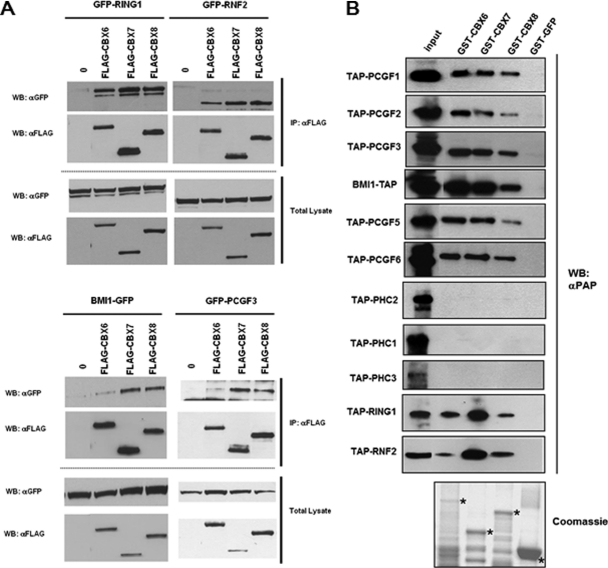
Fig. 2.
Comparison of CBX6, CBX7, and CBX8 interactions with the other PRC1 components. A, Co-immunoprecipitation analyses of interactions between CBX6, CBX7, CBX8, and the RING1, RNF2, BMI1, and PCGF3 components of the PRC1 protein complex show that CBX6 interacts with RING1 and RNF2 in a similar manner as CBX7 and CBX8, whereas CBX6 interaction with BMI1 and PCGF3 is significantly lower. HEK293 were transfected to express transiently FLAG-tagged CBX proteins and GFP-tagged RING1, RNF2 (upper panel), BMI1 and PCGF3 (lower panel). Proteins were immunoprecipitated (IP) with an anti-FLAG antibody linked to agarose beads, separated by SDS-PAGE, and detected (WB) using the indicated αGFP or αFLAG antibodies. Levels of transfections were analyzed in total lysates. B, GST-pull down analysis of interactions between CBX6, CBX7, CBX8, and the RING1, RNF2, BMI1, and PCGF3 components of the PRC1 protein complex showing that CBX6, as well as CBX7 and CBX8 interact with PCGF1, PCGF2, PCGF3, BMI1, PCGF5, PCGF6, RING1, and RNF2 in vitro. None of the CBX proteins shows a direct interaction with the Ph orthologs PHC1, PHC2 and PHC3 in vitro. GST-tagged CBX6, CBX7 and CBX8 proteins as well as control GST-GFP fusion were expressed in E. coli, bound to glutathione-Sepharose, and incubated with in vitro expressed TAP-tagged PRC1 components as indicated. Bound proteins were separated by SDS-PAGE and Western blots probed with peroxidase anti-peroxidase (αPAP) antibodies to reveal TAP-tagged proteins. GST-tagged proteins purified from E. coli are indicated with a star on the Coomassie stained gel following protein separation by SDS-PAGE.