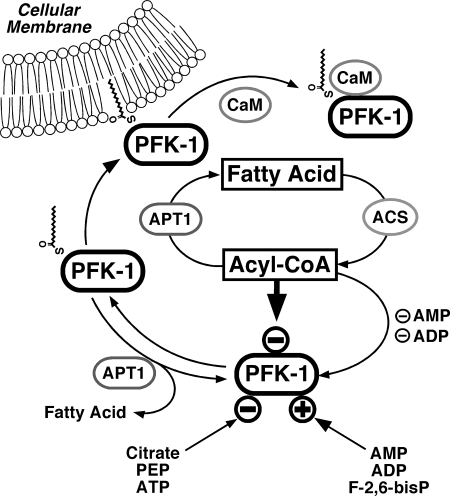
FIGURE 13.
Proposed regulation of glycolysis through fatty acyl-CoA mediated inhibition of PFK-1 and effect of Ca2+/CaM on the membrane compartmentation of acylated PFK-1. Fatty acyl-CoA generated from fatty acids through acyl-CoA synthetases (ACS) inhibit glycolytic flux through phosphofructokinase-1 (PFK-1). Previous work has identified activation of PFK-1 by AMP, ADP, and fructose 2,6-bisphosphate (F-2,6-bisP) and inhibition of PFK-1 through ATP, phosphoenolpyruvate (PEP), and citrate (generated from increased flux of acetyl-CoA through the tricarboxylic acid cycle from β-oxidation of fatty acids). Acyl-protein thioesterase (APT1) reverses fatty acyl-CoA mediated inhibition of PFK-1 either through hydrolysis of noncovalently bound fatty acyl-CoA or through deacylation. Palmitoylation of PFK markedly enhances the binding of calmodulin (CaM) to PFK in a Ca2+-dependent manner and attenuates the binding of palmitoylated PFK to membrane bilayers.