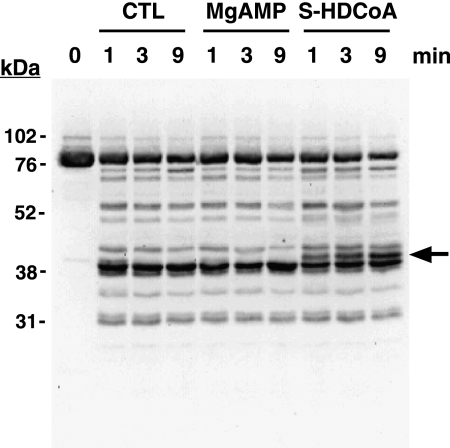
FIGURE 5.
S-Hexadecyl-CoA alters the susceptibility of PFK-1 to proteolysis as determined by tryptic footprinting. Following preincubation of purified rabbit muscle PFK-1 (1 μm, 85 μg/ml) in the presence or absence of 0.25 mm MgAMP or 2.5 μm S-hexadecyl-CoA, sequencing grade modified trypsin was added at a 1:40 w/w ratio and incubated for the indicated times. Trypsinolysis reactions were terminated by addition of 2× SDS-PAGE loading buffer and vortexing. PFK-1 tryptic fragments were resolved by SDS-PAGE, transferred to PVDF membranes, and probed with an antibody directed against the C terminus of PFK-1. Immunoreactive bands were detected by enhanced chemiluminescence using a protein A-horseradish peroxidase conjugate and visualized using a Kodak Image Station as described under “Experimental Procedures.” A novel 42-kDa tryptic peptide generated only in the presence of S-hexadecyl-CoA is indicated by the arrow. Results are representative of three separate experiments. CTL, control.