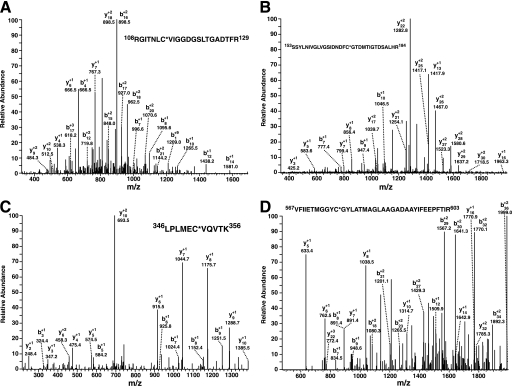
FIGURE 7.
Identification of the sites of palmitoyl-CoA-mediated palmitoylation of phosphofructokinase by mass spectrometry. Purified rabbit skeletal muscle PFK-1 (10 μm) was incubated in the presence or absence of a stoichiometric amount of palmitoyl (Palm)-CoA in 25 mm Tris-HCl, pH 7.5, containing 50 mm KCl and 1 mm DTT for 60 min at 35 °C. Protein samples were precipitated, trypsinized, and processed as described under “Experimental Procedures.” Ion peaks corresponding to predicted palmitoylated tryptic peptides in the full mass scan were selected for product ion analysis. A, product ion mass spectrum of the ion at 2461.34 corresponding to the tryptic peptide (RGITNLC*VIGGDGSLTGADTFR) palmitoylated at Cys-114. B, product ion mass spectrum of the ion at 3609.80 corresponding to the tryptic peptide (SSYLNIVGLVGSIDNDFC*GTDMTIGTDSALR) palmitoylated at Cys-170. C, product ion mass spectrum of the ion at 1498.89 corresponding to the tryptic peptide (LPLMEC*VQVTK) palmitoylated at Cys-351. D, product ion mass spectrum of the ion at 4171.10 corresponding to the tryptic peptide (VFIIETMGGYC*GYLATMAGLAAGADAAYIFEEPFTIR) palmitoylated at Cys-577. Asterisks indicate the sites of palmitoylation. Mass to charge (m/z) ratios of the identified b and y fragment ions are as indicated.