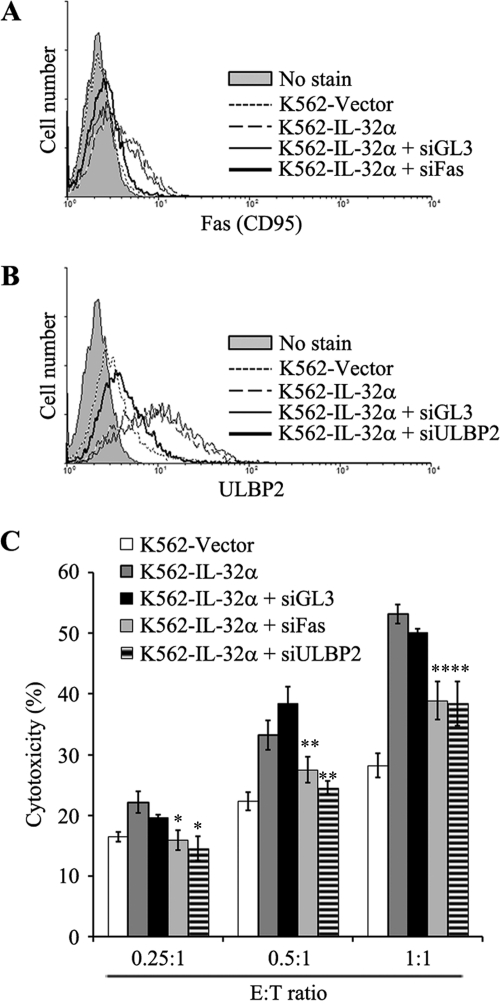
FIGURE 4.
Blocking effect of siRNA against Fas and ULBP2 in IL-32α-induced NK susceptibility in K562 cells. IL-32α-induced Fas and ULBP2 were blocked with specific siRNA. IL-32α-overexpressing K562 cells were transiently transfected with nonrelated control siRNA (GL3 siRNA), Fas siRNA, and ULBP2 siRNA using MicoPorator electroporation. After 48 h, K562 vector, K562-IL-32α, and siRNA-transfected K562-IL-32α (siGL3, siFas, and siULBP2) cells were analyzed. A, Fas expression in K562-vector, K562-IL-32α, and siRNA-transfected K562-IL-32α (siGL3 and siFas) cells. B, ULBP2 expression in K562 vector, K562-IL-32α, and siRNA-transfected K562-IL-32α (siGL3 and siULBP2) cells. C, NK susceptibility test. K562 vector, K562-IL-32α, and siRNA-transfected K562-IL-32α (siGL3, siFas, and siULBP2) cells were used as target cells, and NK-92MI cells were used as effector cells. Target cells were stained with CFSE as described under “Experimental Procedures,” and then the cells were incubated with NK-92MI cells at an optimal E:T ratio (E:T ratio: 0.25:1, 0.5:1, and 1:1). After a 1-h incubation, co-incubated effector cells and target cells were stained with 7-AAD. The killing effect was analyzed by measurement of percentage of CFSE and 7-AAD double-positive region. Data presented are representative of three independent data sets for each condition (*, p < 0.01; **, p < 0.05 versus K562-IL-32α).