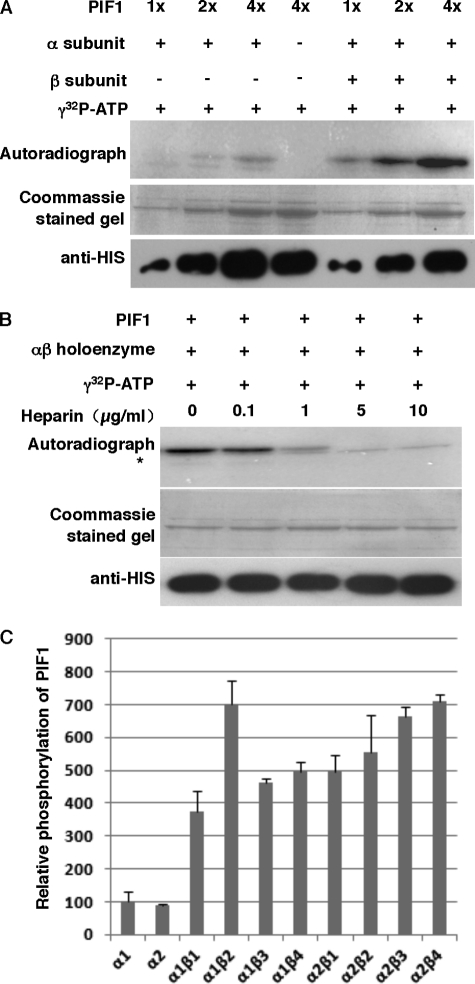
FIGURE 1.
CK2 phosphorylates PIF1 in vitro. A, phosphorylation of PIF1 by CK2 is enhanced by the β subunit. The autoradiogram shows that HIS-PIF1 was strongly phosphorylated by a recombinant CK2 holoenzyme in vitro. CK2 phosphorylation assays were performed in 20 μl of kinase assay mixtures that contained 50 mm Hepes-KOH (pH 7.6), 5 mm MgCl2, 2.4 mm DTT, 100 mm KCl, 0.2 mm γ-[32P]ATP (∼250 cpm/pmol), ∼1 pmol CK2 α or αβ holoenzyme, and ∼10–20 pmol PIF1. The reaction was incubated at 30 °C for 30 min and terminated by the addition of 4× SDS loading buffer. Samples were boiled for 3 min and separated on 10% SDS-PAGE gels. The gels were dried and exposed to a phosphorImager. B, autoradiogram showing that heparin effectively inhibited HIS-PIF1 phosphorylation by CK2 in a dosage-dependent manner. The kinase assays were performed as described in A. The asterisk indicates a nonspecific band. C, different subunit combinations of CK2 differentially phosphorylate PIF1 in vitro. The kinase assays were performed as described in A. Statistical analyses for significant differences are shown in supplemental Table S3.