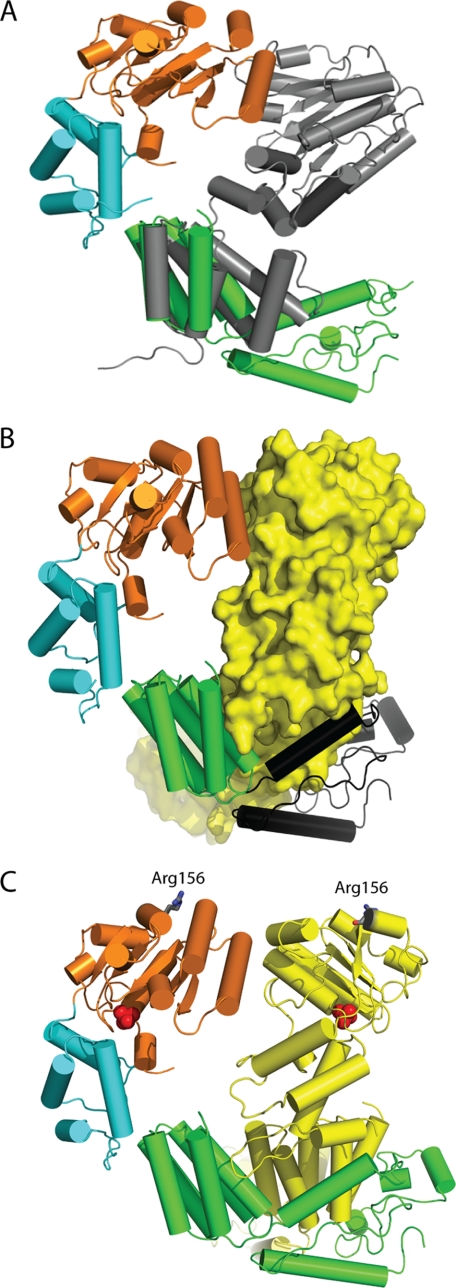
FIGURE 2.
Comparison of MgsA to the bacterial clamp loader. A, overlay of the oligomerization domain of the gamma protein of the E. coli clamp loader (Protein Data Bank 1JR3, chain B) (gray) aligned with the oligomerization domain of MgsA demonstrates that MgsA possesses an additional ∼100 C-terminal residues not present in the clamp loader. B, additional ∼100 residues on the C terminus of MgsA (colored in black) wrap around the neighboring protomer to form an extensive interface. C, Arg finger residues (Arg-156) of neighboring protomers are shown as gray sticks demonstrating the positioning relative to the neighboring ATP-binding site (identified by the phosphate ions, shown as red spheres).