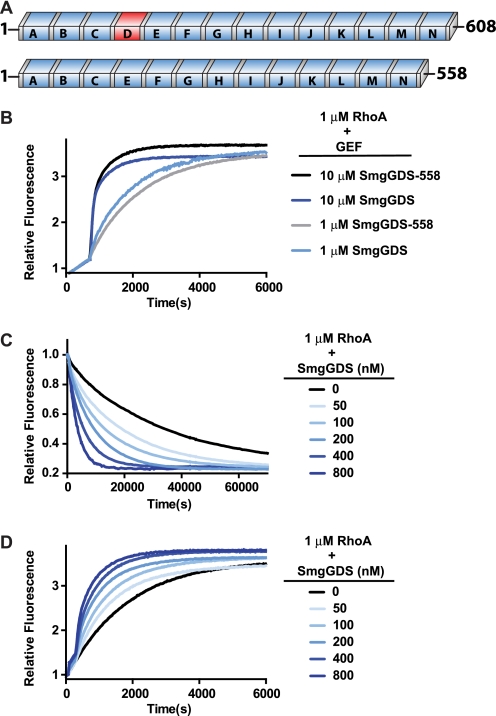
FIGURE 2.
Activation of RhoA by SmgGDS is catalytic and independent of SmgGDS isoform. A, domain architecture of SmgGDS isoforms is shown with conserved ARM repeats (blue boxes) and inserted repeat (red). B, intrinsic exchange of RhoA (1 μm) was measured for 600 s in exchange buffer before stimulation of nucleotide exchange with the indicated concentration of SmgGDS or SmgGDS-558. C, MANT-GDP-loaded RhoA (1 μm) was incubated in unloading exchange buffer for 600 s before addition of SmgGDS at the indicated concentrations to stimulate nucleotide exchange. D, RhoA (1 μm) was incubated in unloading exchange buffer with 400 nm MANT-GDP in place of the free GDP for 600 s before addition of SmgGDS at the indicated concentrations to stimulate nucleotide exchange.