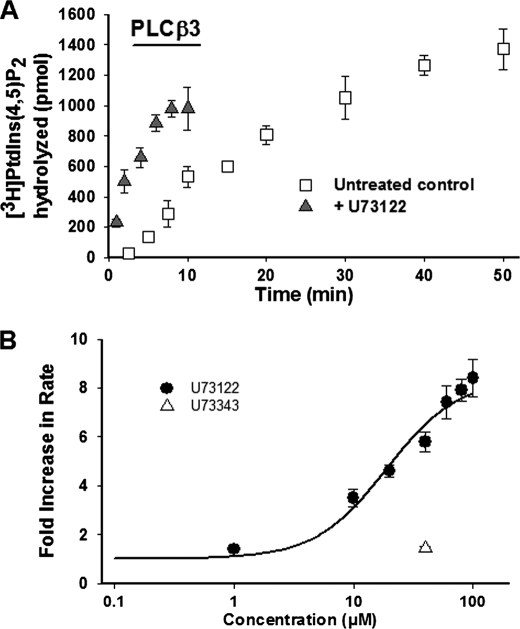
FIGURE 2.
A, time course of PIP2 hydrolysis by hPLCβ3 in DDM-mixed micelles in the presence and absence of U73122 (40 μm). PIP2, reconstituted in DDM, was combined with the assay buffer and hPLCβ3 in a final volume of 100 μl. Assays were initiated by moving samples to a 37 °C water bath and incubating for the indicated times. Incubation times were adjusted to ensure that less than 15% PIP2 was hydrolyzed in all cases. Enzyme hydrolysis rates were determined based on initial time points within the linear phase of activity. Data represent mean ± S.D. from triplicate determinations from one representative experiment. B, concentration-dependent activation of hPLCβ3 by U73122 in DDM-mixed micelles. PIP2, reconstituted in DDM, was combined with assay buffer and hPLCβ3 in a final volume of 100 μl. U73122, at indicated concentrations, was added to the assay mixture prior to the addition of the enzyme. Assays were initiated by moving samples to a 37 °C water bath. Incubation times were adjusted to ensure that less than 15% PIP2 was hydrolyzed in all cases. Data represent mean ± S.D. from triplicate determinations from one representative experiment. EC50(PLCβ3) was determined as described under “Experimental Procedures” and was found to be 19.1 μm in this experiment. The estimated EC50 from these data should be interpreted within the context of the current set of results, as solubility limitations of U73122 in DMSO stock solutions prevented assessment of activation at concentrations greater than 100 μm.