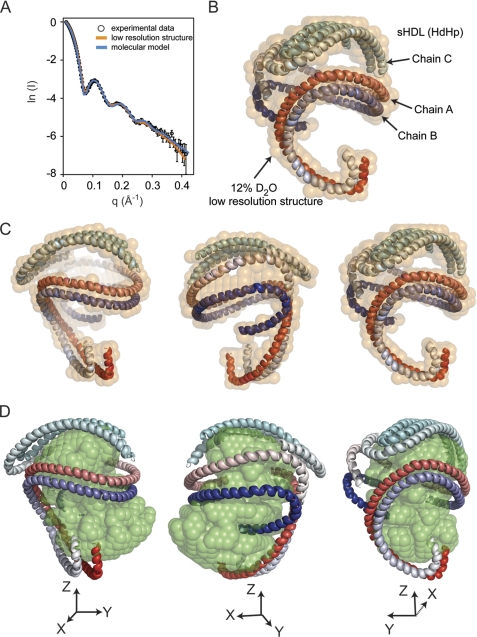
FIGURE 3.
Comparison of experimental neutron scattering intensities with those obtained from the low resolution structure and the molecular model of the apoA1 trimer. A, the fit of scattering intensities produced by the 12% D2O low resolution structure (orange curve) and the molecular model (blue curve) of the apoA1 trimer (HdHp model) to the experimental data. B, the superposition of the molecular model of the apoA1 trimer (HdHp) with the 12% D2O low resolution structure (semitransparent orange beads). The molecular model of the apoA1 trimer is shown as three chains, two of them in a helical dimer conformation, and the third chain forms a hairpin. Because the molecular model does not include any local information about the conformation of the individual amino acid residues, the secondary structure of the chains was assigned to be α-helix for all residues. The three chains are gradient-colored red, blue and cyan. C and D, superposition of the HdHp model with the low resolution structure (orange) of the protein component (12% D2O) of sHDL (C) and the low resolution structure of the lipid component (42% D2O) (D).