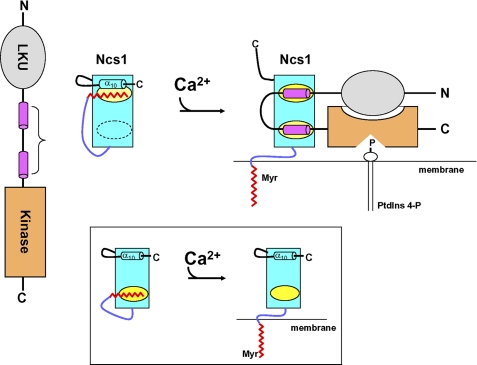
FIGURE 6.
Model of Ca2+-induced activation or PtdIns 4-kinase by Ncs1. Left panel shows schematic diagrams of Pik1 and Ncs1 at low Ca2+. LKU motif (residues 23–98) is in gray; Ncs1-binding region (111–159) is in magenta, and catalytic (kinase) domain (residues 642–916) is in orange. In Ca2+-free Ncs1, an N-terminal arm (purple) places the myristoyl group (red) in a hydrophobic cavity (yellow) flanked by a C-terminal helix (α10). Right panel shows Ca2+-induced conformational changes in Ncs1 that cause exposure of myristate and two hydrophobic patches (yellow), followed by structural rearrangement in Pik1 induced by its binding to Ca2+-bound Ncs1. Ncs1 binding to Pik1 imposes a U-turn in the main chain of Pik1 that is necessary to allow the LKU domain (gray) to interact with the catalytic domain (orange). In addition, N-myristoylation of Ncs1 (red) helps deliver the complex to membranes where it can bind to substrate (PtdIns). Inset shows schematic diagram of a somewhat different Ca2+-myristoyl switch mechanism for recoverin.