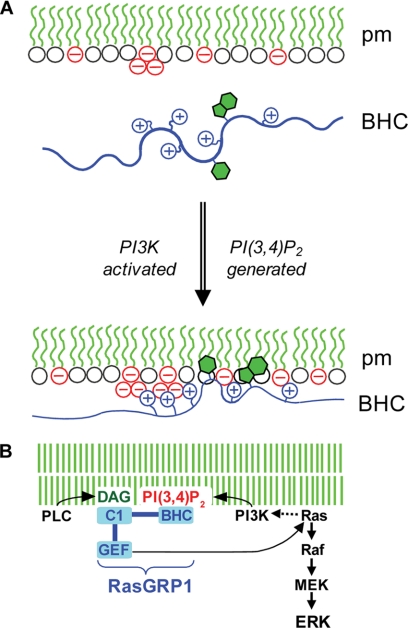
FIGURE 8.
Proposed mechanism of plasma membrane targeting of RasGRP1. A, in the absence of PI3K activation by receptors (top), there is insufficient negative charge at the inner surface of the plasma membrane (pm) to support stable electrostatic binding of RasGRP1 via its BHC, despite the presence of PI(4,5)P2 (triplet of negative charge in the diagram). When PI3K is activated, the phosphoinositide that is generated (PI(3,4)P2 as shown by the second triplet of negative charge or alternatively PI(3,4,5)P3) provides additional clustered negative charges that drive electrostatic binding of the BHC to the membrane, and this enables insertion of aromatic side chains into the bilayer, which further stabilizes binding. B, potential for coincidence detection and signal amplification by RasGRP1. Binding of the BHC to phosphoinositides generated by PI3K could synergize with binding of the C1 domain to DAG generated by PLCγ to maximize plasma membrane targeting of RasGRP1. In addition to triggering downstream effector pathways such as Raf/MEK/ERK, GTP loading of Ras by plasma membrane-localized RasGRP1 could activate PI3K, thus setting up a positive feedback loop amplifying signaling through both the PI3K and Ras pathways.