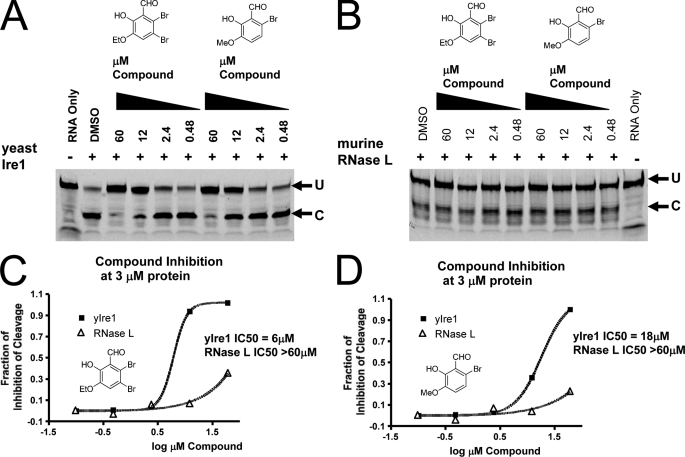
FIGURE 3.
Cross-reactivity analysis of salicylaldehyde analogs against yeast Ire1 and murine RNase L. Both 3-ethoxy-5,6-dibromosalicylaldehyde and 3-methoxy-6-bromosalicylaldehyde displayed cross-reactivity against yeast Ire1 (A). Yeast Ire1-cyto was preincubated with the indicated concentrations of compound for 1 h at room temperature. Then the 5′ FITC-labeled single hairpin RNA substrate (5′-CAUGUCCGCAGCGCAUG-3′) was added and the reaction incubated for 90 min at room temperature. Reaction mixtures were then resolved by PAGE and fluorescence was visualized by a Typhoon imager. Neither 3-ethoxy-5,6-dibromosalicylaldehyde nor 3-methoxy-6-bromosalicylaldehyde show cross-reactivity against murine RNase L (B). A catalytic fragment (residues 333–651) of murine RNase L expressed and purified from bacteria was preincubated with the indicated concentrations of compound for 1 h at room temperature. Then 5′ FITC-labeled RNase L RNA substrate (5′-C11U2C7-3′) was added and the reaction was incubated for 90 min at room temperature. Reaction mixtures were then resolved by PAGE and fluorescence was visualized by a Typhoon imager. IC50 profiles for 3-ethoxy-5,6-dibromosalicylaldehyde (C) and 3-methoxy-6-bromosalicylaldehyde (D) against yeast Ire1 and RNase L are indicated. Quantification of cleavage was performed by phosphorimager analysis and graphed using GraphPad.