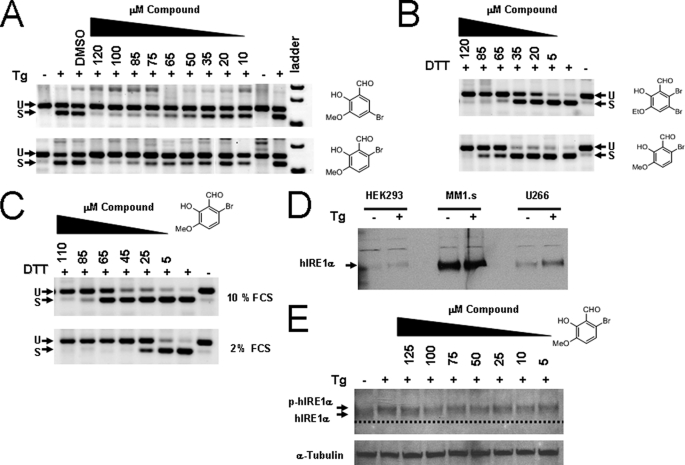
FIGURE 6.
Inhibition of XBP-1 splicing in human cells using salicylaldehyde analogs. HEK293 cells were left untreated or treated with 300 nm Tg for 3 h. Compounds (right of panel) were added 2 h before Tg at the indicated dose; total RNA was harvested and RT-PCR was performed using human-specific XBP-1 primers flanking the splice site 3 h after stress induction. PCR products were run on 4% agarose gels and stained with ethidium bromide and shown as the inverse image. Spliced (S) and unspliced (U) reaction products of XBP-1 mRNA are designated (A). Human myeloma MM1.s cells were treated with 2 mm DTT as the ER stressing agent and exposed to increasing concentrations of the indicated compound for 2 h (B): both DTT and the compounds were added at the same time. When MM1.s cells were treated in 2% FCS medium as in B, the potency of 3-methoxy-6-bromosalicylaldehyde increased, indicating compounds are partially absorbed to serum proteins (C). Western blot showing relative levels of IRE1α in human cell lines both untreated and treated with 300 nm Tg for 2 h (D). MM1.s myeloma cells had high steady state levels of IRE1α compared with HEK293 and the IgE secreting human myeloma cell line U266. When HEK293 cells were incubated with Tg and increasing concentrations of 3-methoxy-6-bromosalicylaldehyde for 3 h, no change in phosphorylation status was observed (E). The dashed line is a reference to observe the slight decrease in mobility due to phosphorylation (p-hIRE1α). The blot was reprobed with anti-α-tubulin antibody used as a loading control.