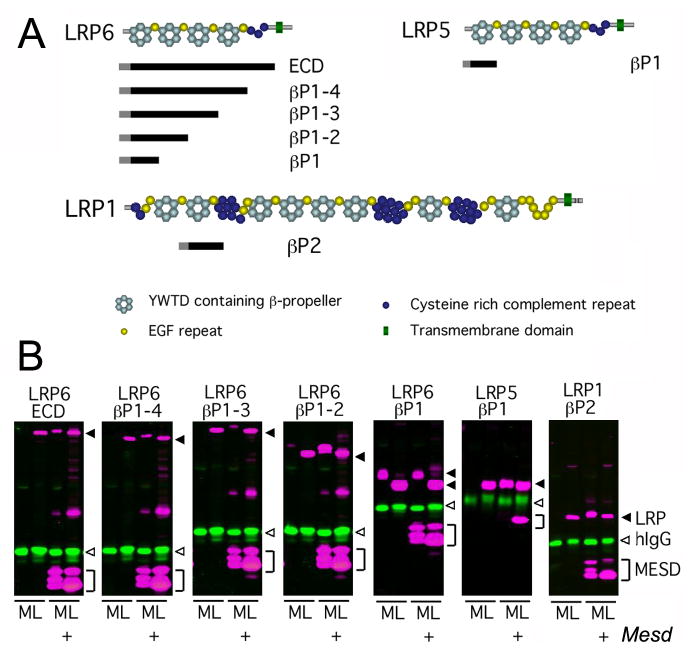
Figure 4. MESD promotes trafficking of β-propeller/EGF domains.
We utilized a soluble receptor secretion assay to identify the minimal LRP domain that requires MESD for maturation. In this assay, soluble truncated receptors were co-transfected with or without Mesd, and the cell lysate (L) and media (M) collected. If the soluble receptor is dependent upon Mesd for maturation, the receptor is expected to accumulate in the cell lysate (L) in the absence of exogenous Mesd. In the presence of exogenous Mesd, the soluble receptor would transit the secretory pathway and be released in to the cell culture media (M). (A) Overview of soluble receptor constructs. Pictured above is a schematic representation of the predicted domain structure of the full length LRP6, LRP5, and LRP1 receptors. The extra-cellular domain (ECD) of LRPs consists of cysteine-rich complement-like repeats (CLRs, dark blue circles), Epidermal growth factor (EGF) repeats (yellow circles), and alternating YWTD containing β-propeller (light blue hexagon) and EGF domains (LRP cartoons adapted from (Strickland et al., 2002)). The black bars located below the full length LRPs indicate the portion of receptor retained in the soluble receptor constructs. The receptor construct name, indicating the β-propeller/EGF domains (βP) included in the construct, is designated to the right of the black bar. Note β-propeller/EGFs are numbered sequentially starting at the N-terminus. All soluble receptors lacked the transmembrane domain (green triangle) present in the full length LRPs, but retained the signal peptide (grey bar) and maintain the juxtaposition of the β-propeller and C-terminal EGF motif (B) Western analysis of soluble LRP5/6 or LRP1 constructs in the presence or absence of MESD. Secreted receptors were detected in the cell culture media (M), and immature receptors were detected in the cell lysate (L). Closed arrowhead, LRP (magenta); open arrowhead, control hIgG (green); bracket, MESD (magenta).