Abstract
The exteroceptive capabilities of the nociceptive system have long been thought to be considerably more limited than those of the tactile system. However, most investigations of spatio-temporal aspects of the nociceptive system have largely focused on intensity coding as consequence of spatial or temporal summation.
Graphesthesia, the identification of numbers “written” on the skin, and assessment of the two-point discrimination thresholds were used to compare the exteroceptive capabilities of the tactile and nociceptive systems. Numbers were “written” on the forearm and the abdomen by tactile stimulation and by painful non-contact infrared laser heat stimulation. Subjects performed both graphesthesia tasks better than chance. The tactile graphesthesia tasks were performed with 89% (82–97%) correct responses on the forearm and 86% (79–94%) correct responses on the abdomen. Tactile graphesthesia tasks were significantly better than painful heat graphesthesia tasks that were performed with 31% (23–40%) and 44% (37–51%) correct responses on the forearm and abdomen, respectively. These findings demonstrate that the central nervous system is capable of assembling complex spatio-temporal patterns of nociceptive information from the body surface into unified mental objects with sufficient accuracy to enable behavioral discrimination.
Keywords: Graphesthesia, Two-point discrimination, Pain cognition, Laser heat stimulation, Spatio-temporal integration
1. Introduction
Within the central nervous system, nociceptive processing is accomplished by a complex interplay of excitatory and inhibitory processes. At the dorsal horn of the spinal cord, inhibitory activity can clearly influence spatial aspects of nociceptive processing. Administration of GABA and glycine antagonists has been shown to enlarge the receptive fields of nociceptive neurons [38,39]. The receptive field size may also vary with central sensitization [7,14]. Such spatial tuning may also be altered in a short-term dynamic fashion. For example, spatial direction of attention has been associated with a shifting and expansion of the receptive field of a nociceptive neuron in the medullary dorsal horn [11] and can substantially alter the spatial integration of nociceptive information [25].
This interplay of excitatory and inhibitory processes is critical for establishing the spatial capabilities of the nociceptive system. Historically, the spatial discriminative capabilities of the nociceptive system have been thought to be considerably poorer than those of the tactile system. However, some discriminative functions, such as single-point localization, are almost equally accurate between tactile and painful heat stimuli, underscoring the exteroceptive capacity of the nociceptive system [15,19,27]. More complex spatio-temporal perceptional phenomena such as perceived spatial distortion of stimuli presented in spatio-temporal patterns, termed the saltation illusion, are also similar in the tactile [9] and nociceptive [31] systems.
To date, the capacity of the nociceptive system to encode spatial information from complex stimuli remains poorly explored as the majority of investigations have focused on the experience of intensity rather than the spatial percept. Multiple noxious stimuli applied to spatially distinct body regions interact considerably, with summation if stimuli are delivered transiently [22,23] or with inhibition if conditioning noxious stimuli are delivered tonically to heterotopic structures [34] or if large areas are activated simultaneously [3]. Consistent with the large distances over which spatial summation can occur, several investigations indicate that two-point discrimination of noxious information is considerably poorer than that of tactile information [18,27,37]. However, the capacity of the nociceptive system to process complex spatio-temporal patterns of painful information and the possible perception of such patterns remain unknown.
Assessing graphesthesia provides one method of exploring the processing of complex spatial information. Graphesthesia is the ability to recognize numbers ‘written’ by tactile pressure on the skin and is a classic neurological test for the capability of a person to perform higher cortical processing of small differences in the direction and position of a moving tactile sensory input [8,13,26]. In order to better understand the exteroceptive capabilities of the nociceptive system, the present investigation compared graphesthesia and two-point discrimination tasks during innocuous tactile stimulation and non-contact painful heat stimulation at the forearm and abdomen.
2. Materials and methods
2.1. Subjects
Two groups of subjects were included in the study. One group, 12 healthy volunteers (seven men, five women) between the ages of 22 and 37 years participated in the study of graphesthesia on the forearm. Another group of 15 healthy volunteers between the ages of 19 and 36 years were added (eleven men, four women) to evaluate graphesthesia on the abdomen and use larger numbers than those used on the forearm. All subjects gave written, informed consent acknowledging that they would experience experimental painful stimuli and that they were free to withdraw from the experiment at any time without prejudice. All the procedures were approved by the local ethics committee (ref. no N-20070029).
2.2. Thermal stimulation and pain assessment
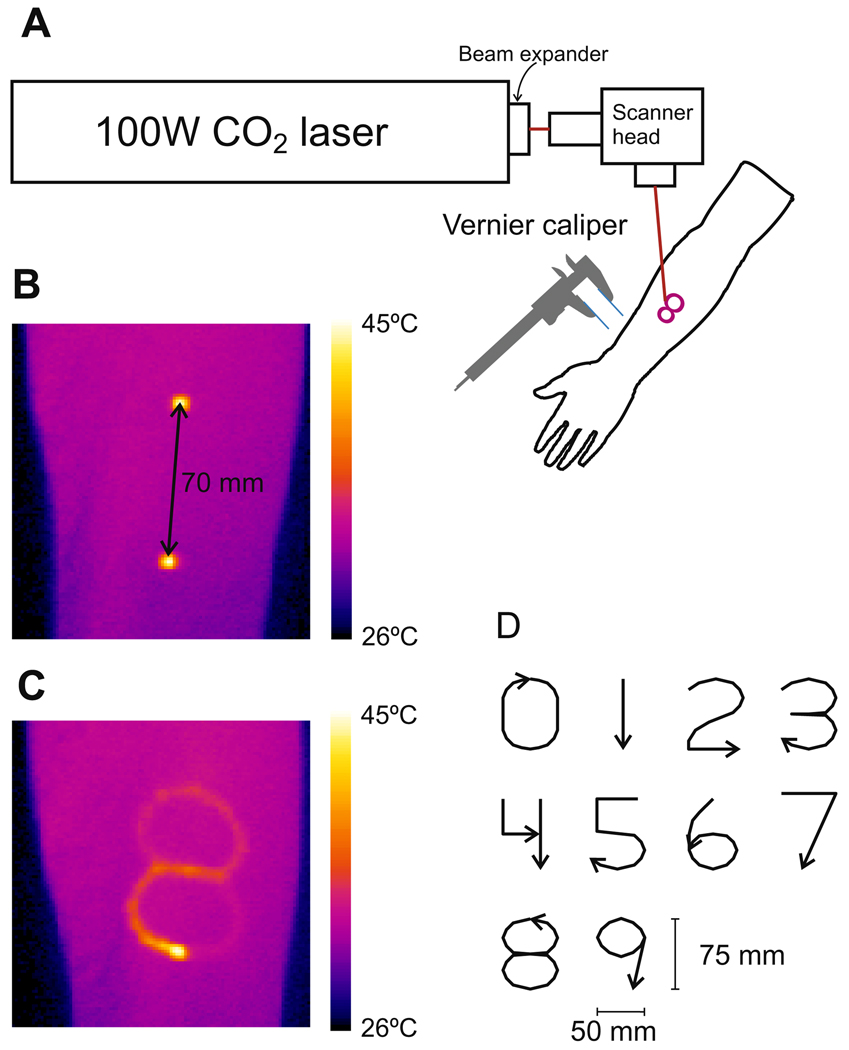
Painful thermal stimuli were delivered to the left and right ventral forearms and the right side of the abdomen by a 100W CO2 laser (Synrad 57-1; Fig. 1). A scanner head (GSI Lumonics General Scanning XY10A) containing two mirrors mounted on galvanometers rapidly, accurately, and reproducibly directed the 4 mm diameter laser beam over the skin. A 1 mm circular dithering was added to the laser trace resulting in a 5 mm diameter gaussian beam. The velocity of the laser movement was kept constant at 35 mm/s. Pilot experiments examining skin surface temperature with an infrared camera (Agema 900, FLIR Systems) confirmed that the skin temperature was elevated in a uniform fashion, see Fig. 1B. Different patterns of stimuli were used to assess two-point discrimination and graphesthesia. Forearm stimulation: two sets of 20 stimulations to assess two-point discrimination and two sets of 10 stimulations to assess graphesthesia were applied to both forearms in a randomized fashion. Abdomen stimulation: two sets of 10 stimulations to assess graphesthesia and two sets of 12 stimulations to assess two-point discrimination were delivered to the right side of the abdomen in a randomized sequence. Stimuli were randomized within all sets and a minimum of 30 s inter-stimulus interval was used for all stimuli.
Fig. 1.
Experimental setup. A CO2 laser was aimed through a scanner head onto the ventral forearm or abdomen of the subject (A). In a two-point discrimination task, two points were stimulated by switching the laser beam rapidly between the points so that the skin temperature was elevated concurrently as imaged by an infrared camera. (B) The laser was shut off when moving between the two points. In a graphesthesia task, the laser beam was slowly moved while stimulating, so that a 75 mm tall number on the forearm and 150 mm tall numbers on the abdomen between 0 and 9 was ‘written’ causing an elevation in the skin temperature as imaged by an infrared camera (C). Two blunt plastic filaments were mounted on a vernier caliper and used to apply non-painful stimulation in the tactile two-point discrimination tasks. The same shape of numbers that were ‘written’ with the laser system was drawn by the index finger of the experimenter in the non-painful tactile graphesthesia tasks by following an aiming beam of the laser to control the writing speed (CO2 laser off; D).
For assessment of painful heat graphesthesia, the scanner head was programmed to draw the numbers 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 on the skin using a font modified from the Schoolhouse Printed A. All numbers were ‘written’ with one contiguous stroke, except the number ‘4’ which was ‘written’ with two separate strokes. The numbers ‘written’ on the forearm were 50 mm wide and 75 mm tall and the bottom of the plane was located proximally and the top distally. The stroke lengths of the numbers were; 0: 203 mm, 1: 75 mm, 2: 180 mm, 3: 214 mm, 4: 143 mm, 5: 179 mm, 6: 182 mm, 7: 132 mm, 8: 265 mm, 9: 168 mm. As the velocity of the beam was constant the stroke lengths were proportional to the stroke durations; 0: 5.8 s, 1: 2.1 s, 2: 5.1 s, 3: 6.1 s, 4: 4.1 s, 5: 5.1 s, 6: 5.2 s, 7: 3.8 s, 8: 7.6 s, 9: 4.8 s.
The numbers ‘written’ on the abdomen were twice as large (100 mm × 150 mm) and the bottom plane was located rostrally on the right side of the abdomen and did not cross the midline. As the study aimed to investigate if graphesthesia is present in the pain system, the size of the numbers on the forearm and abdomen was chosen to be as large as possible while avoiding an angle between the laser beam and the skin to be less than 45°.
A visual analog scale (VAS) was used to assess the pain intensity of the radiant heat stimulations. The scale was anchored by 0 indicating ‘no pain’ and 10 ‘most intense pain imaginable’. For each subject and each stimulus region, stimulus intensity was adjusted separately to the lowest stimulation setting that resulted in a reported VAS rating of more than 1.0 in two consecutive trials. For this procedure an abstract character that extended to all four sides of the coordinate plane was used. For each stimulus presentation, subjects were verbally cued before stimulus onset, and at stimulus offset, were instructed to identify the number in a forced choice task and to provide a VAS rating of average pain intensity during the stimulation.
The method of constant stimuli was used to assess two-point discrimination. Two-point stimuli were separated by 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mm and drawn along the proximal–distal axis of the ventral forearm and were separated by 20, 40, 60, 80, 100, and 120 mm and drawn along the caudal–rostral axis on the abdomen. The scanner head was programmed to draw two separate “points” consisting of a 2 mm line resulting in a ~30 mm2 stimulation area. The laser stimulated one site for 57 ms, then was shut off and rapidly (1525 mm/s) moved to the other site and turned on for 57 ms, shut off and rapidly moved back to the first point. The sequence was repeated 20 times during a 5 s period. This resulted in a sensation of continuous and concurrent stimulation at the two sites. As control stimuli for each of the tested distances a 57 ms delay replaced one of the stimuli, so that only one site was stimulated during the similar 5 s stimulation period. On the forearm, the one-point control stimulus was applied at the distal location for 10, 30, 50, 70, and 90 mm distances and the proximal location for 20, 40, 60, 80, and 100 mm distances. On the abdomen, the one-point control stimuli were applied to the rostral site. The stimulation intensity was the same as that used in the graphesthesia tests. After each stimulation, the subject was asked to rate the perceived number of stimuli (1 or 2) in a forced choice task to provide a VAS rating of pain intensity during the stimulation.
2.3. Tactile stimulation
For assessment of tactile graphesthesia the experimenter drew the numbers with the index finger on the forearm or abdomen of the subject. The size, shape and velocity of the number drawing mimicked that of the heat stimulation by following the aiming beam of the laser (CO2 laser off). The subjects were blinded to the drawing of the number. After each stimulus the subjects were instructed to identify the number in a forced choice task.
For assessment of tactile two-point discrimination, two blunt plastic filaments mounted on a vernier caliper were pressed gently against the skin. Punctuate filaments with an area of 0.8 mm2 were used on the forearm whereas the filaments had an area of 20 mm2 when applied on the abdomen. Similar to the heat stimulation paradigm, one or two points were stimulated in the proximal–distal direction on the forearm and in the caudal–rostral direction on the abdomen for 5 s. The force of the stimulation applied during the tactile graphesthesia and two-point discrimination tasks evoked a clear tactile but painless sensation when applied to the volar forearm of the experiment leader.
2.4. Data analysis
2.4.1. Two-point discrimination
The mean number of points (one or two) perceived by the subject was calculated for each distance when two stimuli were applied. One was subtracted from the mean so that the mean ranged from 0 to 1, zero meaning that one point was perceived and one meaning that two points were perceived. These translated means were fitted to a logistic curve
where the fitting parameter b is the distance at which y = ½ and thus describing the distance at which the subjects were capable of distinguishing two points from one, and the fitting parameter a is the slope at this distance. The receiver operating characteristic (ROC) was calculated and the area under the ROC curve was compared between tactile and painful heat stimulation on the forearm and the abdomen [10].
2.4.2. Graphesthesia
Fischer’s exact test was performed to test a possible association between the number ‘written’ on the skin and the number perceived by the subject. Due to the large numbers of cells in the cross tabulation, a two-sided Monte Carlo approximation of the Fischer’s exact test was performed with a confidence level of 99.9% for the P-values. In order to test if a possible association between the presented number and the perceived number was due to correct responses or systematical misperception, a linear-by-linear test was performed to test if the majority of observations were located in the diagonal of the cross-tabulation ordered by stroke length (8, 3, 0, 6, 2, 5, 9, 4, 7, 1 in descending order). A two-way ANOVA (modality and location as factors) was performed to test if there was a difference in the frequency of correctly perceived numbers. The numbers ‘written’ contain features between the numbers (Fig. 1), e.g., ‘0’ and ‘6’ has the same stroke path until the ascending part where the beam either curves left forming the number ‘6’ or continues forming the number ‘0’. If a subject perceives the initial part but misinterprets the last part of the stroke path it will lead to an incorrect response but a common misperception may be present. To investigate if such a common misperception appeared frequently, the information transfer was calculated for only correct responses and for all responses [13] and compared between modalities and location with an ANOVA test. When calculating information transfer [1], the graphesthesia task was considered as an information channel where a hypothetical receiver would gain information about the actual number ‘written’ based on the response of the subject and the knowledge that only 10 different characters were applied. If a subject performed perfectly by identifying all numbers correctly the transferred information would be log2(10) = 3.3 bits/response. If the subjects were unable to identify the numbers or any features of the numbers and responded at random the information transfer would be 0 bits/response. If a subject can identify all numbers but, e.g., misperceives ‘2’ for ‘3’ and vice versa, the information transfer is still perfect, because the receiver will be able to identify the stimulated number based on the responded number, even though only 80% of the responses would be correct. Misperceptions that are not consequent will only add to the information transfer by the amount that they help the receiver in identifying the stimulated number. For further description and mathematical formula of information transfer during graphesthesia task, please refer to the appendix of [13].
2.4.3. VAS responses
A linear mixed model (one/two stimuli and stimulation side treated as factors and distance as covariate and repeated over subjects) was used to analyze the VAS responses to the painful heat stimuli in the two-point discrimination test. Furthermore, a linear model (stimulation side and distance treated as factors and repeated over subjects) and a Tukey HSD post hoc test were used to find possible differences in the VAS responses when one painful heat stimulus was applied. Potential spatial summation was defined as the VAS response to two stimuli divided by the VAS response to one stimulus. A linear mixed model (stimulation side treated as factors, distance as covariate, and repeated over subjects) was used to analyze if the spatial summation varied significantly over distance. A linear mixed model (stimulation side treated as factor, stroke length of the presented number treated as covariate, and repeated over subjects) was performed to analyze the VAS responses to painful heat graphesthesia test.
Results were summarized as means and 95% confidence intervals. P < 0.05 was considered as significant results.
3. Results
3.1. Two-point discrimination
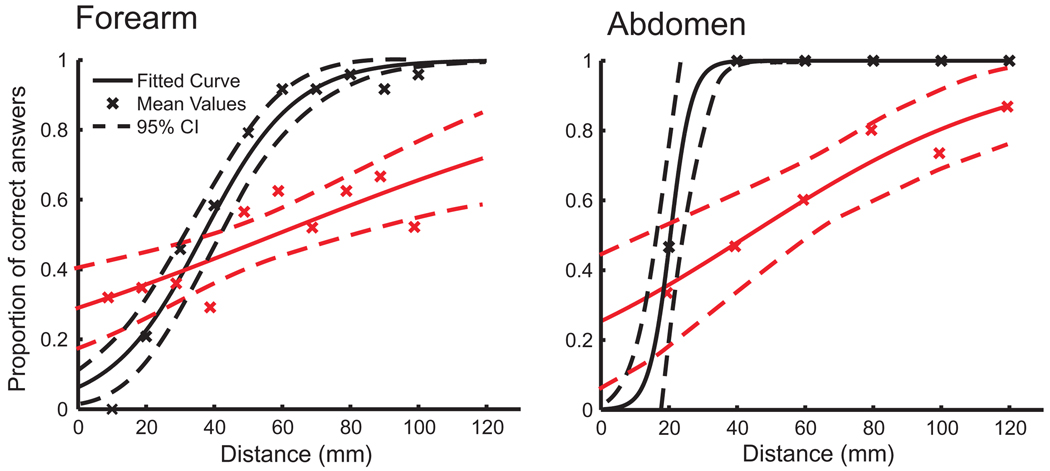
The two-point discrimination distance was longer for painful heat tasks than tactile tasks both on the forearm and on the abdomen (significant by non-overlapping 95% CI; Table 1; Fig. 2). The tactile two-point discrimination distance was shorter on the abdomen than on the forearm (significant by non-overlapping 95% CI), but the difference between painful heat two-point discrimination distances on the forearm and abdomen was not significant (overlapping 95% CI).
Table 1.
Results from the two-point discrimination task as mean and 95% confidence intervals.
| Forearm | Abdomen | ||
|---|---|---|---|
| Threshold (mm) | Tactile | 35.0 (31.4–38.6) | 20.3 (16.2–24.68) |
| Heat | 58.9 (42.0–75.8) | 44.3 (24.6–64.0) | |
| Area under the ROC curve | Tactile | 0.89 (0.84–0.94) | 0.96 (0.92–1.00) |
| Heat | 0.62 (0.55–0.69) | 0.72 (0.61–0.83) | |
| Pain intensity for 1 point | Heat | 1.5 (1.3–1.8) | 2.0 (1.7–2.2) |
| Pain intensity for 2 points | Heat | 2.6 (2.3–2.9) | 2.3 (2.0–2.6) |
Fig. 2.
Two-point discrimination thresholds. The proportions of correct answers after two points were stimulated by tactile (black) or painful heat (red) stimuli indicated by crosses. The data were fitted to a logistic function (solid line) with the 95% confidence interval (95% CI; dashed line). Please note that differences in the tactile two-point discrimination threshold may be due to different probe sizes.
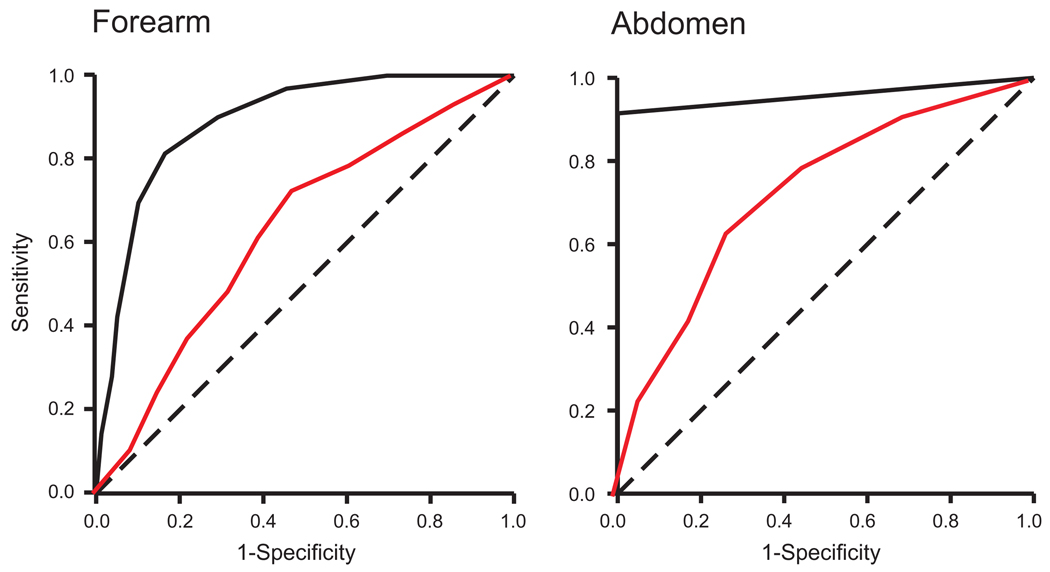
The areas under the ROC curves were larger in the tactile heat tasks than in the painful tasks; both in forearm and abdominal tasks (non-overlapping 95% CI; Table 1; Fig. 3). The areas under the ROC curves were not different between the forearm and the abdominal tasks; neither for tactile nor painful heat tasks (overlapping 95% CI). The areas under the ROC curves were significantly larger than 0.5 for all two-point discrimination tasks (P < 0.001). This indicates that the subjects performed better than if they were merely guessing.
Fig. 3.
Receiver operating characteristic (ROC) curves. The area under the ROC curve was larger for tactile (black) and painful heat (red) two-point discrimination tasks on the forearm and the abdomen (solid line) were all significantly better than merely guessing (dashed diagonal).
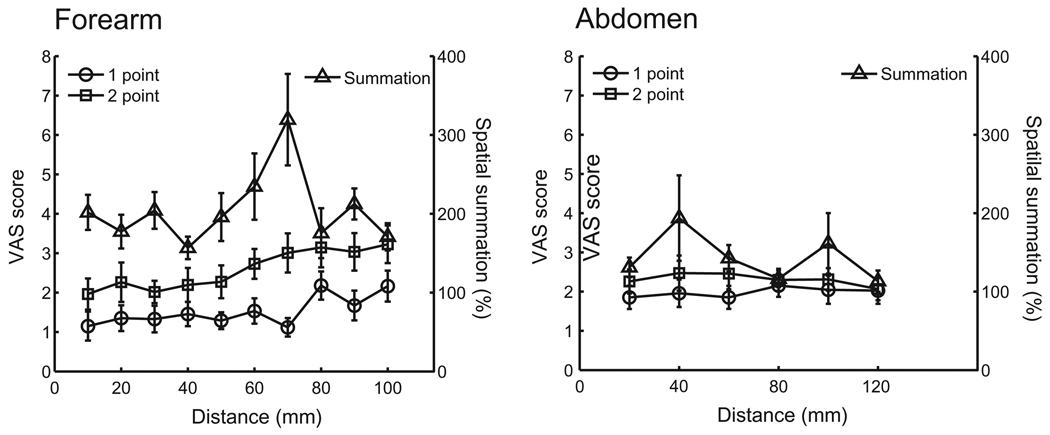
The subjects rated the pain intensity to two-point stimulations significantly higher than one-point stimulations, both on the forearm and the abdomen (mixed linear model; P < 0.001; Table 1; Fig. 4). Furthermore, the spatial summation was additive on the forearm as pain intensity to two stimuli was reported to be 203% (183–223%) of the pain intensity to one stimulus (Fig. 4). The spatial summation was also significant but sub-additive on the abdomen as pain intensity to two stimuli was reported to be 143% (119–167%) of the pain intensity to one stimulus. The spatial summation did not vary with distance on the forearm or on the abdomen (mixed linear model; P > 0.05).
Fig. 4.
Pain intensity during the two-point discrimination task. Pain intensity rated on a visual analog scale (VAS) to painful heat stimulation applied to one (circle) or two points (square). Spatial summation (triangle) was found both on the forearm and the abdomen when both points were stimulated simultaneously.
Furthermore, the pain intensity ratings correlated positively with the distance between the points when two points were stimulated on the forearm (Fig. 4; mixed linear model; P < 0.0001). The mean pain ratings provided after one stimulation on the forearm at some distal sites (10, 30, 50, or 70 mm) were lower than the mean pain ratings to one stimulation at the same proximal sites (80 or 100 mm; linear model; Tukey HSD post hoc; P < 0.05), indicating lower sensitivity towards the hand. There was no significant dependence between distances and pain intensity ratings when stimulation was applied to the abdomen (mixed linear model P > 0.05).
3.2. Graphesthesia
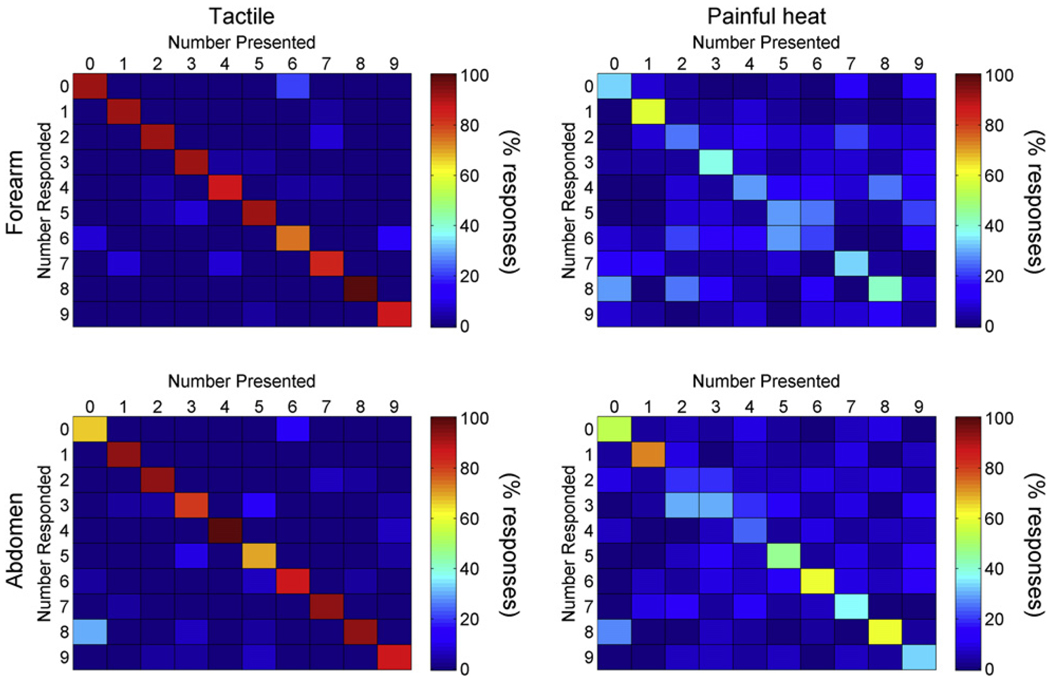
There was a significant association between the actual number presented and the number perceived by the subject both for tactile and painful heat stimulation on the skin of the forearm and the abdomen (Fig. 5; Fischer’s exact; P < 0.001). A statistically significant linear-by-linear association of the cross-tabulation ordered by stimulation length was found for stimulations on both the forearm and the abdomen and for both tactile and painful heat stimulation (P < 0.001).
Fig. 5.
Illustration of perceived numbers ordered by the number presented. Cross tabulation of association between actual numbers presented on the forearm (upper panel) and the abdomen (lower panel) and numbers perceived by the subjects. The color bars indicate the percentage of number responded for each number presented.
Subjects perceived the correct number more frequently and the information transfer was higher when the number was ‘written’ by tactile stimulation than by painful heat stimulation both on the forearm and the abdomen (two-way ANOVA and Tukey, P < 0.001; Table 2). There was no significant association between the stimulated forearm and the number of correct responses (Chi Square, P = 0.5). Increasing the size of the written numbers by stimulation at the abdomen instead of the forearm did not significantly increase the number of correct responses in the tactile (P = 0.9) nor in the painful heat trials (P = 0.07), however, the information transfer was higher for larger numbers when applied by painful heat (P < 0.05) but not by tactile (P > 0.05, three-way ANOVA and Tukey) stimulation. The information transfer was higher when not only correct responses but also all responses were considered for painful heat (P < 0.001), but not for tactile (P > 0.05, three-way ANOVA and Tukey) stimulations.
Table 2.
Results from the graphesthesia task as mean and 95% confidence intervals. The information transfer of correct responses in the graphesthesia tasks was higher in the tactile tasks than the painful heat tasks. Adding trials increased the information transfer for the painful heat (P < 0.001) but not for tactile tasks (P > 0.05, three-way ANOVA and Tukey).
| Forearm | Abdomen | ||
|---|---|---|---|
| Probability of correct response (%) | Tactile | 89 (82–97) | 86 (79–94) |
| Heat | 31 (23–40) | 44 (37–51) | |
| Information transfer of correct responses (bits/response) | Tactile | 3.1 (3.0–3.3) | 3.1 (3.0–3.2) |
| Heat | 1.5 (1.1–2.0) | 2.1 (1.8–2.3) | |
| Total information transfer (bits/response) | Tactile | 3.2 (3.1–3.3) | 3.2 (3.1–3.3) |
| Heat | 2.5 (2.4–2.6) | 2.7 (2.6–2.8) |
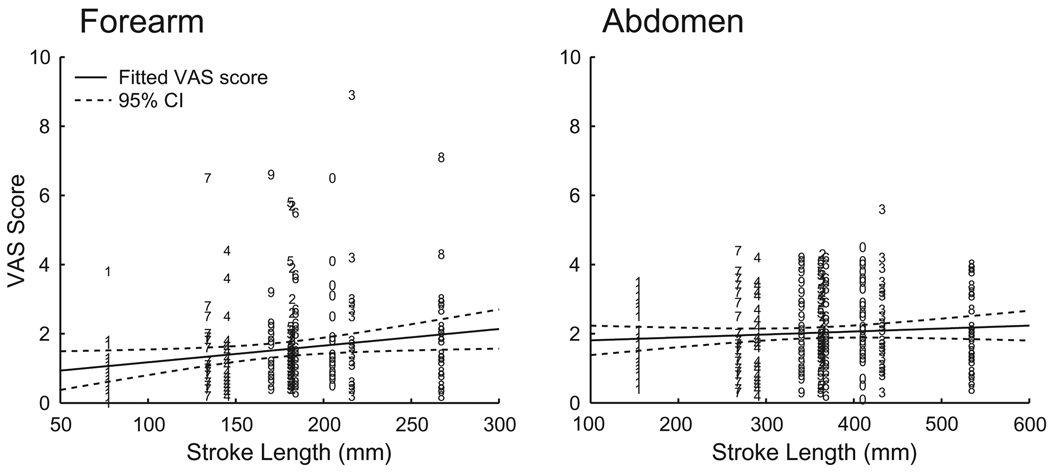
There was a significant correlation between the stroke length and the pain intensity rating when painful heat stimulations were applied to the forearm (linear regression, P < 0.005, R2 = 0.032) but not when applied to the abdomen (linear regression, P = 0.2; Fig. 6).
Fig. 6.
Scatter plot of stroke length vs. pain intensity. Each visual analog scale (VAS) score is indicated by the number ‘written’. There was a significant correlation between pain intensity rating and the stroke length of the number when stimulations were applied to the forearm (linear regression, P < 0.005, R2 = 0.032) but not when applied to the abdomen (linear regression, P = 0.19); fitted line represented in solid, 95% confidence interval (CI) represented in dashed line).
Comparing the VAS responses between trials where the subjects answered correctly and trials where they answered wrongly did not show any significant differences regardless of whether the painful heat stimuli were applied to the forearm or the abdomen. Similarly, there were no significant differences in VAS responses between stimuli applied to the dominant or the non-dominant arm (linear model; P > 0.05).
4. Discussion
During the graphesthesia task, subjects were able to recognize numbers ‘written’ on the skin with painful laser stimulation. These findings indicate that the central nervous system is capable of transforming complex spatial patterns of nociceptive information into unified objects with sufficient accuracy to allow behavioral discrimination. Broader spatial and temporal tuning of nociceptive processing may account for the greater difficulty of the painful heat vs. tactile graphesthesia task.
4.1. Spatial discrimination and spatial summation
Spatial discrimination of noxious stimuli has typically been examined by simultaneous two-point discrimination using contact thermodes, and, even with potential confounds arising from low-threshold mechanoreceptive input from the probe touching the skin, the threshold was observed as 50–100 mm on the forearm assessed with a 9 cm2 thermode [6]. The noxious two-point discrimination threshold was considerably larger than that observed with tactile stimulation as 17.3 mm by Martikainen and Pertovaara [18] and 40 mm by Weinstein [33]. In the present investigation, potential tactile confounds were eliminated by using laser stimulation to evoke pain (beam spot area of 30 mm2). Consistent with these previous studies, painful heat two-point discrimination thresholds were larger than thresholds observed by tactile stimuli.
During the two-point discrimination task, considerable spatial summation was observed. Subjects may have used this information deliberately or non-deliberately to decide the number of stimulation sites if they were in doubt, however, remembering that the one/two-point stimulation sequence was randomized. This may have resulted in a better performance in the two-point discrimination test across all distances. But, the degree of spatial summation did not vary significantly across separation distance, while subjects’ performance improved considerably as stimulus separations were increased. In contrast to previous studies that concluded that spatial discrimination and spatial summation were mutually exclusive [6], the present findings indicate that, over a limited range (60–120 mm separation distances), spatial summation can co-exist with spatial discrimination.
For the graphesthesia task, a similar issue may have potentially influenced the ability of subjects to identify the smaller scaled set of numbers on the forearm. As the drawing velocity was kept constant the total drawing time was different between the numbers, e.g., the drawing time of the number ‘1’ was shorter than the drawing time of the number ‘8’. Longer stimulation time and larger stimulation area may have led to spatial [22] and temporal summation [21] of the nociceptive input. The relatively high ratio of correct answers to presentation of the numbers ‘1’ and ‘8’ may indicate that the subjects used the drawing time and/or the pain intensity to identify the written number (Fig. 5). However, the correlation coefficient indicated that the stroke length was only able to explain approximately 3% of the variation of the VAS rating to the smaller scale numbers delivered to the forearm (Fig. 6). This correlation was not seen in the larger scale numbers delivered in the abdominal tasks where the subjects performed even better, so the influence of such a confound was most likely minimal.
Although an inter-stimulation interval of at least 30 s between heat stimulations was used a possibility of skin sensitization remains. This may have ‘blurred’ the perceptual abilities of the subjects, as sensitization is most likely associated with enlarged receptive fields [16]. The discriminative abilities of the pain system may therefore have been underestimated. However, as random sequences were used, any bias from sensitization on the individual numbers was avoided.
The frequency of correctly identified numbers was above chance and several common misperception pairs of numbers occurred (Fig. 5). The information transfer showed that common misperception pairs contain a significant amount of common information in the painful heat graphesthesia tests (Table 2). Therefore, in some trials, subjects perceived sufficient features of the ‘written’ number to exclude some numbers without necessarily being able to recognize the correct number.
4.2. Spatial tuning
The substantial spatial summation of pain intensity observed during the two-point discrimination task and the larger two-point discrimination distance confirm that mechanisms supporting nociceptive processing have a broad spatial tuning, as the physically separated stimuli evoke neural activity which interacts at some level of the neuroaxis. At the neuronal level, complex interactions of excitatory and inhibitory factors determine the spatial response properties of the neuronal population. The spatial distribution of activity may be extended by the spatial organization of the dendritic arbors of nociceptive neurons, by propagation of afferent information via propriospinal interneurons and by primary nociceptive afferents arborizing after they enter the spinal cord [30,36].
Conversely, inhibition through either local or descending mechanisms can substantially shrink the receptive fields of dorsal horn neurons [4,38,39]. In particular, lateral inhibition has long been known to enhance the two-point discrimination capability in the tactile system [20]. Lateral inhibition in the nociceptive system has not been studied extensively. However, wide dynamic range neurons typically have an on-center off-surround receptive field organization [12] which would typically support lateral inhibition. In withdrawal reflex pathways, excitatory and inhibitory receptive reflex fields have been observed with relationship to the biomechanical efficacy of a specific muscle [29]. The excitatory reflex receptive fields probably reflect receptive field properties of wide dynamic range neurons, [28] whereas the inhibitory neuronal mechanisms are still undisclosed. Substantial overlap between the excitatory and inhibitory reflex receptive fields has been observed which also support broad tuning in the nociceptive system. Thus, the relatively large distances needed before the inhibitory surround is stimulated and overlap between excitatory and inhibitory receptive fields would also contribute substantially to the broad spatial tuning of nociceptive processing and could partially explain the poorer performance in the painful heat two-point discrimination and graphesthesia tasks.
In addition to being broadly tuned, the spatial response profiles of nociceptive processing appear to be asymmetric. When only one heat stimulus was applied during the two-point discrimination task on the forearm, the VAS responses were perceived as more intense at proximal stimulation locations than at distal stimulation points if the points were separated by more than 50 mm. This is in line with the distally directed inhibition found by Quevedo and Coghill [24], and may relate to the asymmetric inhibitory receptive field of wide dynamic range neurons [12] or higher sensitivity at proximal areas [17]. If such a distal inhibition was present during two-point stimulation the sensation of the distal point may have been masked by the sensation of the proximal point, and thus biasing the subjects to perceive that only one point was stimulated. Moreover, such distally directed inhibition could significantly complicate the cognitive assembling of the information during the graphesthesia task, as only portions of the drawn figures would be subjectively available.
Many critical features of the ‘written’ numbers were considerably smaller than the two-point discrimination thresholds but were still recognized. However, since one-point localization capabilities are roughly the same between tactile stimuli and noxious thermal stimuli (typically 22–24 mm on the forearm [32]), it appears unlikely that differences in spatial tuning can fully account for differences in graphesthetic abilities. Difference in surround inhibition and temporal aspects of the stimulation pattern seem to be fundamental to the graphesthetic abilities as recognition of the direction of a drawn line has been shown to be essential to the graphesthetic abilities [2].
4.3. Temporal tuning
Differences in temporal tuning of the processing of tactile vs. nociceptive information may also play a significant role in determining the accuracy of graphesthesia. For example, noxious stimulus offset may take nearly 3 s to detect [35] while detection of innocuous stimulus offset occurs considerably faster [5]. Using a two-point discrimination paradigm that presented each point sequentially following a variable delay, Ylioja et al. [37] determined that spatial discrimination improved significantly as the delay between the stimuli increased to greater than 3 s. Since graphesthesia is, in part, a dynamic sequential single-point localization task enabling the subject to translate a temporal sequence of movement across the skin into a complex spatial pattern [2], the apparent spatial acuity would be significantly influenced by the temporal tuning of sensory processing.
5. Conclusions
The present data indicate that the central nervous system has the capacity to process highly complex spatio-temporal patterns of purely nociceptive information and underscore the exteroceptive capabilities of the nociceptive system. These exteroceptive capabilities may be crucial for the identification of the nature of external threats to body integrity and the subsequent optimization of escape strategies. Deficits in exteroceptive capabilities may accompany unbalanced interactions between excitatory and inhibitory mechanisms in spatio-temporal perception of pain. In chronic pain states, such unbalanced interactions may lead to pain spread, pain mislocalization, and sensitivation. A deeper understanding of the spatio-temporal processing and cognition of pain may lead to a better understanding of pain pathology.
Footnotes
Conflict of interest
The authors have no conflict of interest to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.pain.2010.05.016.
References
- 1.Ash RB. Information theory. New York: Wiley; 1990. [Google Scholar]
- 2.Bender MB, Stacy C, Cohen J. Agraphesthesia: a disorder of directional cutaneous kinesthesia or a disorientation in cutaneous space. J Neurol Sci. 1982;53:531–555. doi: 10.1016/0022-510x(82)90249-0. [DOI] [PubMed] [Google Scholar]
- 3.Bouhassira D, Gall O, Chitour D, Le Bars D. Dorsal horn convergent neurones: negative feedback triggered by spatial summation of nociceptive afferents. Pain. 1995;62:195–200. doi: 10.1016/0304-3959(94)00270-O. [DOI] [PubMed] [Google Scholar]
- 4.Bremner LR, Fitzgerald M. Postnatal tuning of cutaneous inhibitory receptive fields in the rat. J Physiol (Lond) 2008;586:1529–1537. doi: 10.1113/jphysiol.2007.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JI, Ha B, Bushnell MC, Pike B, Duncan GH. Differentiating noxious- and innocuous-related activation of human somatosensory cortices using temporal analysis of fMRI. J Neurophysiol. 2002;88:464–474. doi: 10.1152/jn.2002.88.1.464. [DOI] [PubMed] [Google Scholar]
- 6.Defrin R, Givon R, Raz N, Urca G. Spatial summation and spatial discrimination of pain sensation. Pain. 2006;126:123–131. doi: 10.1016/j.pain.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Dubner R. Neuronal plasticity in the spinal and medullary dorsal horns – a possible role in central pain mechanisms. Pain Central Nerv Syst Dis. 1991:143–155. [Google Scholar]
- 8.Freeman C, Okun MS. Origins of the sensory examination in neurology. Semin Neurol. 2002;22:399–407. doi: 10.1055/s-2002-36762. [DOI] [PubMed] [Google Scholar]
- 9.Geldard FA, Sherrick CE. Cutaneous rabbit – perceptual illusion. Science. 1972;178:178–179. doi: 10.1126/science.178.4057.178. [DOI] [PubMed] [Google Scholar]
- 10.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 11.Hayes RL, Dubner R, Hoffman DS. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. II. Behavioral modulation of responses to thermal and mechanical stimuli. J Neurophysiol. 1981;46:428–443. doi: 10.1152/jn.1981.46.3.428. [DOI] [PubMed] [Google Scholar]
- 12.Hillman P, Wall PD. Inhibitory and excitatory factors influencing receptive fields of lamina-5 spinal cord cells. Exp Brain Res. 1969;9:284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KO, Phillips JR. Tactile spatial resolution. I. Two-point discrimination, gap detection, grating resolution, and letter recognition. J Neurophysiol. 1981;46:1177–1192. doi: 10.1152/jn.1981.46.6.1177. [DOI] [PubMed] [Google Scholar]
- 14.Kauppila T, Mohammadian P, Nielsen J, Andersen OK, Arendt-Nielsen L. Capsaicin-induced impairment of tactile spatial discrimination ability in man: indirect evidence for increased receptive fields in human nervous system. Brain Res. 1998;797:361–367. doi: 10.1016/s0006-8993(98)00431-4. [DOI] [PubMed] [Google Scholar]
- 15.Koltzenburg M, Handwerker HO, Torebjörk HE. The ability of humans to localise noxious stimuli. Neurosci Lett. 1993;150:219–222. doi: 10.1016/0304-3940(93)90540-2. [DOI] [PubMed] [Google Scholar]
- 16.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Petrini L, Defrin R, Madeleine P, Arendt-Nielsen L. High resolution topographical mapping of warm and cold sensitivities. Clin Neurophysiol. 2008;119:2641–2646. doi: 10.1016/j.clinph.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Martikainen IK, Pertovaara A. Spatial discrimination of one versus two test stimuli in the human skin: dissociation of mechanisms depending on the task and the modality of stimulation. Neurosci Lett. 2002;328:322–324. doi: 10.1016/s0304-3940(02)00555-4. [DOI] [PubMed] [Google Scholar]
- 19.Moore CEG, Schady W. Cutaneous localisation of laser induced pain in humans. Neurosci Lett. 1995;193:208–210. doi: 10.1016/0304-3940(95)91313-7. [DOI] [PubMed] [Google Scholar]
- 20.Mountcastle VB, Darian-Smith I. Neural mechanisms in somesthesia. In: Mountcastle VB, editor. Medical physiology. Vol. 1968. Mosby: St. Louis; vol. II. pp. 1372–1423. [Google Scholar]
- 21.Nielsen J, Arendt-Nielsen L. The importance of stimulus configuration for temporal summation of first and second pain to repeated heat stimuli. Eur J Pain. 1998;2:329–341. doi: 10.1016/s1090-3801(98)90031-3. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen J, Arendt-Nielsen L. Spatial summation of heat induced pain within and between dermatomes. Somatosens Motor Res. 1997;14:119–125. doi: 10.1080/08990229771123. [DOI] [PubMed] [Google Scholar]
- 23.Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–1279. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 24.Quevedo AS, Coghill RC. An illusion of proximal radiation of pain due to distally directed inhibition. J Pain. 2007;8:280–286. doi: 10.1016/j.jpain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Quevedo AS, Coghill RC. Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci. 2007;27:11635–11640. doi: 10.1523/JNEUROSCI.3356-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitan RM, Wolfson D. Halstead–Reitan neuropsychological test battery: theory and clinical interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 27.Schlereth T, Magerl W, Treede RD. Spatial discrimination thresholds for pain and touch in human hairy skin. Pain. 2001;92:187–194. doi: 10.1016/s0304-3959(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 28.Schouenborg J, Weng HR, Kalliomaki J, Holmberg H. A survey of spinal dorsal horn neurons encoding the spatial-organization of withdrawal reflexes in the rat. Exp Brain Res. 1995;106:19–27. doi: 10.1007/BF00241353. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenborg FA, Andersen OK, Arendt-Nielsen L. Modular organization of excitatory and inhibitory reflex receptive fields elicited by electrical stimulation of the foot sole in man. Clin Neurophysiol. 2000;111:2160–2169. doi: 10.1016/s1388-2457(00)00472-7. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- 31.Trojan J, Stolle AM, Kleinbohl D, Morch CD, Arendt-Nielsen L, Holzl R. The saltation illusion demonstrates integrative processing of spatiotemporal information in thermoceptive and nociceptive networks. Exp Brain Res. 2006;170:88–96. doi: 10.1007/s00221-005-0190-z. [DOI] [PubMed] [Google Scholar]
- 32.Trojan J, Kleinböhl D, Stolle AM, Andersen OK, Hölzl R, Arendt-Nielsen L. Psychophysical ‘perceptual maps’ of heat and pain sensations by direct localization of CO2 laser stimuli on the skin. Brain Res. 2006;1120:106–113. doi: 10.1016/j.brainres.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: Kenshalo DR, editor. The skin senses. Springfield: Charles C Thomas; 1968. pp. 195–222. [Google Scholar]
- 34.Willer JC, Roby A, Le Bars D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain. 1984;107:1095–1112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]
- 35.Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 2008;134:174–186. doi: 10.1016/j.pain.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yezierski RP, Culberson JL, Brown PB. Cells of origin of propriospinal connections to cat lumbosacral gray as determined with horseradish peroxidase. Exp Neurol. 1980;69:493–512. doi: 10.1016/0014-4886(80)90047-3. [DOI] [PubMed] [Google Scholar]
- 37.Ylioja S, Carlson S, Raij TT, Pertovaara A. Localization of touch versus heat pain in the human hand: a dissociative effect of temporal parameters on discriminative capacity and decision strategy. Pain. 2006;121:6–13. doi: 10.1016/j.pain.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Yokota T, Nishikawa N, Nishikawa Y. Effects of strychnine upon different classes of trigeminal subnucleus caudalis neurons. Brain Res. 1979;168:430–434. doi: 10.1016/0006-8993(79)90188-4. [DOI] [PubMed] [Google Scholar]
- 39.Yokota T, Nishikawa Y. Action of picrotoxin upon trigeminal subnucleus caudalis neurons in the monkey. Brain Res. 1979;171:369–373. doi: 10.1016/0006-8993(79)90345-7. [DOI] [PubMed] [Google Scholar]