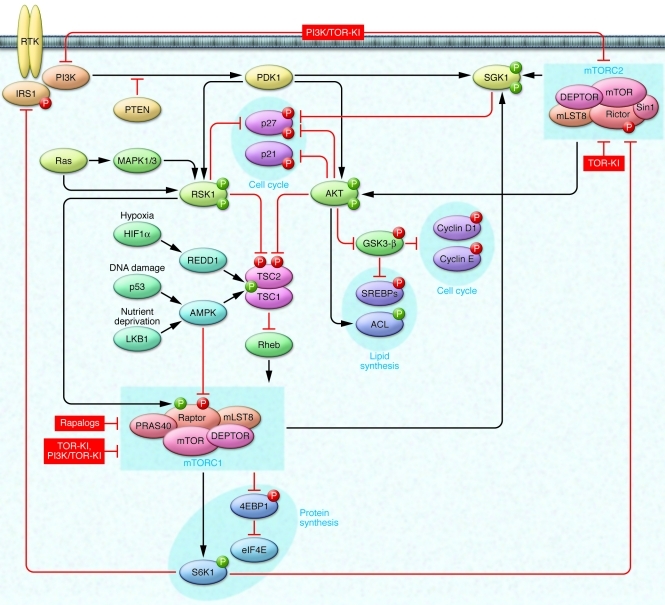
Figure 1. Targeting the mTOR signaling network for cancer therapy.
mTOR-based targeting strategies are presented in the context of the PI3K/mTOR signaling network. Pathways activating mTOR via RTKs and PI3K are shown together with effectors regulating protein and lipid biosynthesis and cell cycle. mTORC1 and mTORC2 modulate cell cycle via effects on Cdk inhibitors p21 and p27, cyclin D1, and cyclin E; SREBPs and ACL regulate lipid biosynthesis downstream of AKT; mTORC1 phosphorylates 4EBP1 and S6K1 to activate critical drivers of global protein translation. Also represented are important feedback pathways whereby mTORC1 reduces signaling through PI3K and mTORC2: S6K1 phosphorylates IRS1, promoting its proteolysis; S6K1 phosphorylates Rictor to inhibit mTORC2-dependent AKT activation. The TSC1/2 complex serves as a relay center for tumor microenvironmental queues. Oncogenic PI3K/PDK1 and Ras/MAPK signaling cooperate to reduce TSC1/2 activity. Hypoxia (via HIF1α), DNA damage (via p53), and nutrient deprivation (via LKB1) all activate TSC1/2 to restrain mTORC1 and biosynthetic processes in normal tissue. These pathways are often inactivated during tumorigenesis. Rapalogs are mTORC1-specific inhibitors. TOR-KIs more potently inhibit both mTOR complexes. Dual PI3K/TOR-KIs additionally block upstream signaling via PI3K. Green circles represent stimulatory phosphorylations; red circles, inhibitory phosphorylations.