Table 1.
Optimization of C-H Alkylation
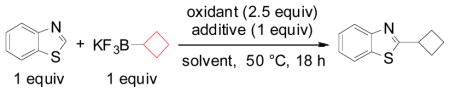 | ||||
|---|---|---|---|---|
| entry | oxidant | additive | solvent | GCMS conversion |
| 1 | Mn(OAc)3 | H2SO4 | AcOH:H2O 1:1 | 71% |
| 2 | Cu(OAc)2 | H2SO4 | AcOH:H2O 1:1 | 1% |
| 3 | KMnO4 | H2SO4 | AcOH:H2O 1:1 | 20% |
| 4 | Ce(SO4)2 | H2SO4 | AcOH:H2O 1:1 | 54% |
| 5 | K2Cr2O7 | H2SO4 | AcOH:H2O 1:1 | 26% |
| 6 | Fe(SO4)2•7H2O | H2SO4 | AcOH:H2O 1:1 | 6% |
| 7 | (NH4) 2S2O7 | H2SO4 | AcOH:H2O 1:1 | 12% |
| 8 | benzoquinone | H2SO4 | AcOH:H2O 1:1 | 3% |
| 9 | Mn(OAc)3 | H2SO4 | AcOH:H2O 1:1 | 6% |
| 10 | Mn(OAc)3 | TFA | AcOH:H2O 1:1 | 78% (60%)a |
| 11 | Mn(OAc)3 | TFAb | AcOH:H2O 1:1 | 71% |
| 12 | Mn(OAc)3 | KHF2 | AcOH:H2O 1:1 | 71% |
| 13 | Mn(OAc)3 | - | AcOH:H2O 1:1 | 65% |
| 14 | Mn(OAc)3 | TFA | AcOH | 48% |
| 15 | Mn(OAc)3 | TFA | DMSO | 4% |
| 16 | Mn(OAc)3 | TFA | CH3CN | 41% |
| 17 | Mn(OAc)3 | TFA | MeOH | 54% |
| 18 | Mn(OAc)3 | TFA | ClCH2CH2Cl | 51% |
| 19 | Mn(OAc)3 | TFA | acetone | 31% |
Reaction performed at room temperature.
Reaction performed with only 0.2 equiv of trifluoroacetic acid.
