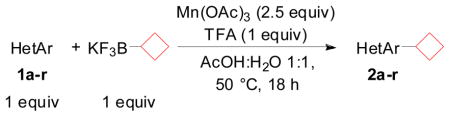
Table 2.
Scope of Coupling with Diverse Heteroaryls
 | |||
|---|---|---|---|
| entry | heteroaryl | yield (%)a | |
| 1 | 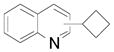 |
2aa, 2ab C2:C4 (70:30) | 44 (58) |
| 2 | 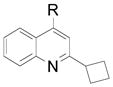 |
2b R= Me 2c R= Cl |
65 (83) 56 (81) |
| 3 | 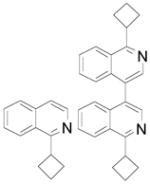 |
2da 2db |
54% + 17% (92) |
| 4 | 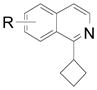 |
2e R= 3-CO2Me 2f R= 4-Br 2g R= 5-Br |
59 (75) 61 (80) 64 (82) |
| 5 | 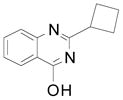 |
2h | 59 (72) |
| 6 | 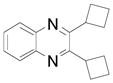 |
2i | 59 (81)b |
| 7 | 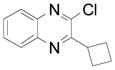 |
2j | 61 (70) |
| 8 | 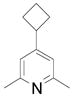 |
2k | 34 (77) |
| 9 | 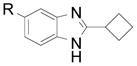 |
2l R: H 2m R: Me |
60 (63) 60 (70) |
| 10 | 2n | 54 (76) | |
| 11 | 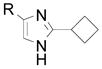 |
2o R= H 2p R= Br 2q R= Ph |
31 (93) 42 (54) 34 (66) |
| 12 | 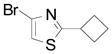 |
2r | 11 (74) |
Heterocycle (1.0 mmol), potassium cyclobutyltrifluoroborate (1.0 mmol), Mn(OAc)3 (2.5 mmol), TFA (1.0 mmol), AcOH/H2O 1:1 (0.08 M), 50 °C, 18 h.
Isolated yields and conversions (indicated in parentheses) determined by 1H NMR spectroscopic analysis of the crude mixture.
Heterocycle (1.0 mmol), potassium cyclobutyltrifluoroborate (3.5 mmol), Mn(OAc)3 (5.0 mmol), TFA (1.0 mmol), AcOH/H2O 1:1 (0.08 M), 50 °C, 18 h.
