Abstract
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome, which is caused by mutation of the autoimmune regulator (AIRE) gene, is a highly variable disease characterized by multiple endocrine failure, chronic mucocutaneous candidiasis, and various ectodermal defects. AIRE is a transcriptional regulator classically expressed in medullary thymic epithelial cells, monocytes, macrophages, and dendritic cells. Previous studies have suggested that AIRE can shuttle between the nucleus and cytoplasm of cells, although its cytoplasmic functions are poorly characterized. Through mass spectrometry analysis of proteins co-immunoprecipitating with cytoplasmic AIRE, we identified a novel association of AIRE with the intermediate filament protein cytokeratin 17 (K17) in the THP-1 monocyte cell line. We confirmed AIRE expression in HaCaT epidermal keratinocytes, as well as its interaction with K17. Confocal microscopy of human fetal and adult scalp hair follicles demonstrated a cytoplasmic pattern of AIRE staining that moderately colocalized with K17. The cytoplasmic association of AIRE with the intermediate filament network in human epidermal and follicular keratinocytes may provide a new path to understanding the ectodermal abnormalities associated with the APECED syndrome.
The autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome is characterized by development of autoimmunity toward select endocrine tissue (mostly in the form of adrenocortical failure and/or hypoparathyroidism), an immune deficiency resulting in chronic candidiasis, as well as various ectodermal disorders that include tooth enamel hypoplasia, vitiligo, alopecia, and nail dystrophy.1 Since the formal classification of the disease,2 a single gene has been identified as causative and encodes the Autoimmune Regulator (AIRE) protein.3 The AIRE protein contains several key domains, including two plant homeodomain (PHD) zinc fingers, four LXXLL motifs, a Sp100, AIRE-1, NucP41/75, DEAF-1 SAND domain, and a recently identified Caspase Recruitment Domain (CARD) at the N-terminus,4 the last of which has been shown to be a mutational hotspot in AIRE that affects its transactivating potential.5
Prior structural and subcellular analyses of AIRE have demonstrated its strong nuclear localization with a high DNA binding capacity. These characteristics are thought to allow AIRE to transcriptionally modulate ectopic peripheral tissue antigen expression in medullary thymic epithelial cells. In this capacity, AIRE facilitates T cell negative selection through ensuring the full and appropriate spectrum of self-antigen expression in the thymus.5 Other roles for AIRE, however, have been raised as a feature of its biology. Exogenously expressed AIRE-GFP or wild-type AIRE in COS-1 and Hela cells demonstrates that AIRE has the capacity to traffic between the nucleus and cytoplasm.6 Although the functional significance of extranuclear AIRE remains unknown, it can localize to intermediate filaments and/or microtubules6–8 in the cytoplasm.
Although a plausible mechanism is in place for the role of AIRE in promoting autoimmunity, there has been little research regarding the pathogenesis of ectodermal dystrophy (ED) and chronic candidiasis in APECED patients. Aberrant adaptive immunity in APECED has been proposed to result in the immunodeficiency with Candida susceptibility,9 and the ectodermal manifestations10,11 have been considered secondary. However, other studies have demonstrated AIRE expression in monocytes, macrophages, and dendritic cells,12 suggesting that AIRE defects in other cell lineages may contribute to pathogenesis. The current study began in an effort to characterize the role of AIRE in monocytes, but led to the unexpected finding of an association between AIRE and the intermediate filament protein cytokeratin 17 (K17). This association was further investigated in human skin, where we identified the expression of AIRE in epidermal keratinocytes and an association of AIRE with K17 in both HaCaT cells and hair follicles of the human scalp. Our findings indicate that AIRE has a broader expression profile than was previously appreciated and raises the possibility for novel mechanisms underlying AIRE-associated ectodermal disorders.
Materials and Methods
Cell Lines, Tissue, and Antibodies
The THP-1 cell line stably expressing a secreted embryonic alkaline phosphatase (SEAP) reporter construct under the control of an NF-κB promoter (THP-1 Blue; InvivoGen, San Diego, CA) was grown in RPMI supplemented with 2 mmol/L l-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mmol/L HEPES, 1.0 mmol/L sodium pyruvate, penicillin, and streptomycin. Medium was supplemented with 200 μg/ml Zeocin to maintain selection of the reporter construct. HaCaT cells were grown in DMEM supplemented with 10% Fetal Bovine Serum, 2 mmol/L l-glutamine, 1.0 mmol/L sodium pyruvate, penicillin, and streptomycin. For experiments with human tissue, this study used only normally discarded human scalp obtained anonymously from plastic surgical procedures through the Cooperative Human Tissue Network and was approved by the University of Pennsylvania's Institutional Review Board office as an exempt protocol. Fetal human tissue was obtained from Advanced Bioscience Resources (Alameda, CA). Antibodies used had specificities against the following human proteins: AIRE-1 (rabbit polyclonal H-300, used in confocal microscopy and THP-1 cell immunoprecipitation; goat polyclonal D-17, used in immunblot), cytokeratin 17 (K17) (mouse monoclonal E3, used in confocal microscopy, and rabbit polyclonal EPR1624Y, used in co-immunoprecipitation). All aforementioned antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) with the exception of the rabbit polyclonal antibody against human K17, which was obtained from Abcam (Cambridge, MA); HRP-conjugated anti-mouse Ig and HRP-conjugated anti-rabbit Ig (GE Health care, Piscataway, NJ), and HRP-conjugated anti-goat Ig (Santa Cruz) were used as secondaries in Western blotting.
Analysis of AIRE Expression, Putative AIRE Interacting Proteins, and Subsequent Co-Immunoprecipitation
To study constitutive interacting proteins with AIRE, THP-1 cells (1.5E7) were lysed in 1 mL lysis buffer (200 mmol/L NaCl, 1% NP-40, 10 mmol/L Tris-HCl [pH 7.5], 5 mmol/L EDTA, and 10% glycerol) supplemented with protease and phosphatase inhibitors. Cytoplasmic fractions obtained after centrifugation of whole cell lysates were incubated with 20 μg/ml of AIRE-specific IgG-coated or IgG isotype control–coated protein G agarose (Invitrogen, Carlsbad, CA) for 2 hours. After incubation with lysate, beads were washed thrice and bound proteins eluted with NuPage sample buffer and run on a NuPAGE SDS gel (Invitrogen). The gel was subsequently stained with Colloidal Coomassie (Invitrogen) according to the manufacturer's instructions to identify unique proteins in the anti-AIRE precipitate compared to isotype precipitate. The gel was evaluated in the Protein Core Facility of the Children's Hospital of Philadelphia and the protein band at ∼50 kDa studied using vacuum matrix-assisted laser desorption (vMALDI) mass spectrometry as previously described.13 Reciprocal co-immunoprecipitation was performed using a similar procedure with HaCaT cells (5E6 cells/condition) as described previously20 and analyzed by Western blot using the appropriate antibodies described in Cell Lines, Tissue, and Antibodies. RNA was isolated from HaCaT, THP-1, and COS-1 cells using the RNAEasy Kit (Qiagen, Valencia, CA) and cDNA synthesized using Applied Biosystems' High Capacity cDNA synthesis kit (Applied Biosystems, Carlsbad, CA). Applied Biosystems Taqman Gene expression assay (assay ID: Hs00230833_m1) was used to assess the presence of AIRE, and expression was compared to cDNA from THP-1 cells (AIRE-positive control) and COS-1 cells (AIRE-negative control),7 with concurrent amplification of actin used as an amplification control. DNA was resolved on a 1% agarose gel.
Immunocytochemistry, Immunofluorescence, and Confocal Microscopy
0.1E6 HaCaT cells were plated on culture slides (VWR, Radnor, PA) and incubated overnight. Human scalp sections (both adult and fetal sections, the latter at 16 weeks of gestation) were prepared fresh from surgical procedures and embedded in OCT, and cryosections prepared (8 to 10 μm thickness) and used for immunofluorescence. For both scalp sections and HaCaT cells thereafter, slides were rinsed in PBS, fixed and permeabilized with 4% paraformaldehyde containing 0.1% Triton X-100 and 0.2% saponin, washed in PBS, and then incubated with anti-AIRE H-300 or pre-immune rabbit IgG (Novozymes, Davis, CA) at equal concentrations, followed by AlexaFluor647-conjugated goat anti-rabbit (Invitrogen). Cells were then washed again and incubated with mouse monoclonal anti-K17 E3 followed by subsequent incubation with FITC-conjugated goat anti-mouse IgG (Invitrogen) and mounting with Vectashield medium (H-1200) containing DAPI.
Confocal microscopy was performed as described20 using a Zeiss Z1 with a Yokogawa CSU-10 spinning disk, a Zeiss 63x Plan-Achrochromat 1.43NA objective (or a Zeiss 10× Acroplan 0.25NA objective) (Carl Zeiss, Jena, Germany) and four independent laser lines controlled using a Spectral Photonics LMM5 laser merge module (Spectral Applied Research, Richmond Hill, ON, Canada). Specific fluorescence was detected using Chroma narrow band-pass filters (Chroma Technology Corp., Rockingham, VT) and a Hamamatsu R2 monochromatic camera (Hamamatsu Photonics KK, Hamamatsu, Japan). Microscope settings were established using IgG-stained cells to define true positive signal from nonspecific fluorescence and cells individually stained with each fluorescent reagent to minimize bleedthrough. Once optimal settings were obtained, they were maintained constant throughout the experiment. For HaCaT cells, z-stacks of 0.2 μm thickness were taken for all imaged fields and imaging performed using three lasers through individual x,y planes throughout the total z axis, which was established by setting upper and lower z-limits of the cell of interest; figures show representative x,y planes from these z axis image series. For skin sections, two-dimensional images were taken across multiple fields, and each figure shows a representative field. Voxel or pixel colocalization scatter plots and PCCs were generated using Improvision Volocity Software (PerkinElmer, Waltham, MA), and fluorescent regions defined using intensity thresholds, which were applied consistently to all cells evaluated. Pixel scatter plots from all representative images were generated with images taken at ×63 magnification.
Results
Biochemical Discovery and Assessment of Association between AIRE and K17
To investigate the role of AIRE in the extrathymic environment, we initially focused on human monocytes, given their previously defined AIRE expression profile and known roles in defense against Candida.12 Thus, we used the THP-1 monocytic cell line to perform a search for potential proteins that may constitutively associate with AIRE. Experiments to identify interacting proteins by co-immunoprecipitation yielded several candidates (Figure 1A). A substantive protein band just below 50 kDa appearing exclusive to the AIRE immunoprecipitate was identified and then excised, digested, and analyzed by mass spectrometry13. Unexpectedly, extensive peptide coverage therein defined the presence of K17 (Figure 1B). K17 is an intermediate filament protein important for maintenance of epithelial cell cytoarchitecture and is expressed in nails and hair follicles.14 Mutations in the K17 gene are associated with a distinct ectodermal disorder, pachyonychia congenita type II.15 Given certain clinical similarities between pachyonychia congenita type II and the ectodermal abnormalities found in some APECED syndrome patients, we further investigated the potential interaction of AIRE with K17 in human keratinocytes. Initially, we used the HaCaT keratinocyte cell line to evaluate this interaction because it had been previously reported to express K17.16 We first determined the expression of AIRE in the HaCaT cell line by using reverse-transcription polymerase chain reaction (RT-PCR) (Figure 1C). To confirm an association between AIRE and K17 in HaCaT cells, co-immunoprecipitation followed by immunoblot analysis was performed with specific anti-AIRE and anti-K17 antibodies. K17 was identified in AIRE immunoprecipitate (Figure 1D, left) and AIRE in K17 immunoprecipitate (Figure 1D, right). Thus, AIRE is expressed in HaCaT cells and biochemically associates with K17.
Figure 1.
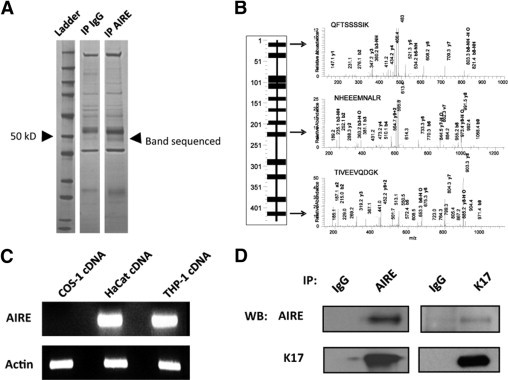
Identification of AIRE association with K17. A: THP-1 cytoplasmic lysate was immunoprecipitated with either rabbit anti-AIRE or rabbit-IgG, resolved on an SDS gel, and then stained with Colloidal Coomassie. A band appearing specific to AIRE below 50 kD was excised, trypsin-digested, and subjected to mass spectrometric analysis. IP, immunoprecipitate. B: Twelve unique tryptic peptides for K17 were identified and their positioning in a schematic of the protein is displayed (left). The arrows demonstrate examples of the K17-specific peptide ion spectra. Assigned β- and γ-ions are labeled. C: RT-PCR analysis of AIRE message in HaCaT cells, with THP-1 monocytes used as a positive control, and COS-1 cells as a negative control, for AIRE expression. Actin is showed as positive control for amplification. D: Confirmatory immunoprecipitation of HaCaT whole-cell lysate with either rabbit anti-human AIRE or rabbit anti-human K17, followed by Western blot (WB) for AIRE (with goat anti-AIRE antibody) or K17 (with rabbit anti-K17 antibody). Isotype immunoprecipitations were performed as negative controls.
Subcellular Localization of AIRE and K17 by Confocal Microscopy
To further elucidate the interaction between K17 and AIRE in HaCaT cells, confocal microscopy was performed to determine their subcellular localization (Figure 2A). When grown overnight on culture slides, the HaCaT cells were uniformly spread (Figure 2A) with a filamentous pattern of K17. As expected, AIRE was also localized within the nucleus as defined by the region of DAPI fluorescence. AIRE was additionally identified in a cytoplasmic filamentous pattern sharing distribution with K17. To better visualize colocalization between AIRE and K17, a scatter plot quantifying individual pixel intensities was generated (Figure 2C, left) and defined a representative Pearson's correlation coefficient (PCC) of 0.698. Thus, in a keratinocyte cell line, endogenous AIRE was present outside of the nucleus and was in a filamentous arrangement substantially colocalized with K17.
Figure 2.
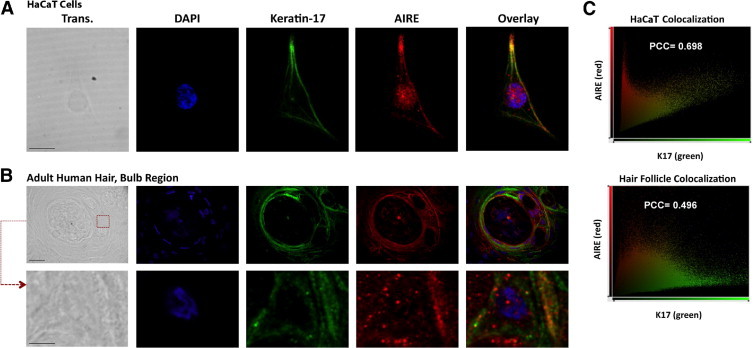
Confocal microscopy demonstrates AIRE and K17 colocalization in HaCaT cells and adult human hair follicles. Individual or overlaid fluorescent images of DAPI (nucleus, blue), K17 (green), or AIRE (red) are shown for HaCaT keratinocytes (A) or human hair tissue (B). A: AIRE demonstrates expected nuclear localization with an additional cytoplasmic, filamentous localization distal to the nucleus. Scale bar = 10 μm. B: AIRE expression in human hair follicles (bulb region shown), where it partly colocalizes with K17 (green) in an extranuclear, filamentous arrangement. A region of tissue at ×63 magnification is further magnified (bottom) to show subcellular localization. The regions of interest chosen for higher magnification are identified in the transmitted light image (Trans.) with the red (×63 image) dashed box. Scale bars = 20 μm (×63 image), and 5 μm (bottom), respectively. C: Representative colocalization scatter plots demonstrating the voxel or pixel intensity for AIRE (red, y axis) and K17 (green, x axis) for HaCaT cells (left) and adult human hair follicles (right) from the images shown in A and B, respectively, with representative Pearson's correlation coefficients (PCC).
To determine whether extranuclear expression of AIRE was present in primary human tissue and thus potentially germane to the ED of APECED, we evaluated AIRE expression in human hair follicles, a tissue type that is known to express K17 in the outer root sheath epithelia.17 Initial staining in the bulb region of hair follicles demonstrated expression of AIRE (Figure 2B, top). Overlap with some K17-containing regions was observed, with a representative PCC of 0.496 (Figure 2C, right). Enlargement of a single cell within the tissue revealed predominantly cytoplasmic AIRE localization, with both a filamentous and speckled arrangement of AIRE (Figure 2B, bottom). To better define AIRE expression patterns in human hair follicles, AIRE and K17 were evaluated in adult telogen and anagen hair follicles. As expected, K17 expression was detected in the follicular root sheath epithelia, most prominently in the outer layers. Extranuclear AIRE expression was detected throughout most of the outer root sheath epithelia (Figure 3A). Higher magnification demonstrated moderate colocalization of AIRE and K17, with PCCs of 0.203 (anagen) and 0.435 (telogen) (Figure 3A). Substantial staining of the surrounding hair matrix was not detected (Figure 3A, ×10 images).
Figure 3.
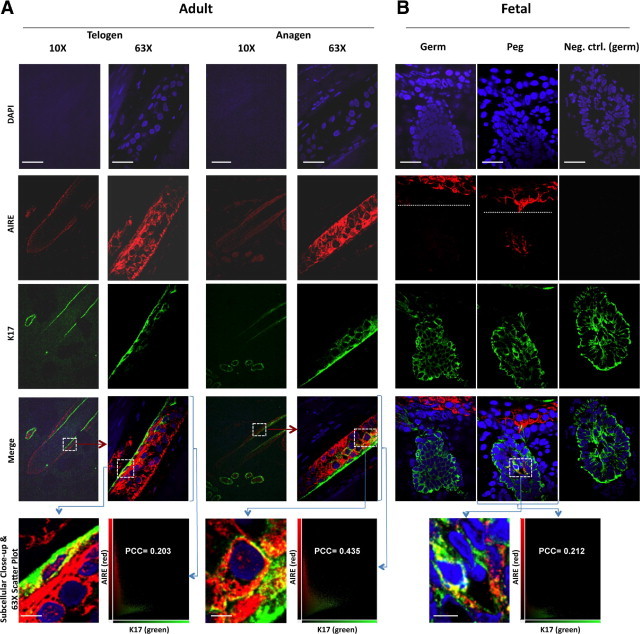
Evaluation of AIRE in postnatal hair cycling and hair follicle development. Individual or overlaid fluorescent images for DAPI (blue), K17 (green), or AIRE (red) are shown in adult telogen and anagen hair follicles (A) and 16-week-old human fetal scalp (B). A: AIRE expression in the outer root sheath of both telogen (left) and anagen (right) hair follicles, with some colocalization with K17 (subcellular close-up). White boxes indicate areas of magnification from ×10 to ×63 (red arrow) and from ×63 to subcellular close-up (blue arrow). B: AIRE expression in the nonfollicular epidermis and developing follicular pegs, but not the primary hair germ in human fetal scalp. White dashed lines in the AIRE channel delineate the epidermal/dermal border. K17 expression shown in both primary germ and follicular peg; subcellular magnification with AIRE colocalization (bottom). Rabbit isotype control (right) is included to show negative staining comparison to primary germ for AIRE. White box indicates area of close-up. Representative colocalization scatter plots with associated PCCs are shown corresponding to the ×63 image indicated in A and B. Scale bar = 150 μm for ×10, 25 μm for ×63, and 10 μm for subcellular close-ups shown in A and B.
To further define whether AIRE is expressed during hair follicle development, AIRE and K17 were evaluated in human 16-week-old fetal scalp sections. K17 showed robust expression in all of the developing hair follicle germs and pegs (Figure 3B). AIRE showed robust expression in the nonfollicular epidermis, and focal expression in the developing follicular pegs, but not in primary hair germs. As in adult hair follicles, there was some AIRE and K17 co-localization (seen with subcellular close-up) with a representative PCC of 0.212 in the follicular peg (Figure 3B). The staining for AIRE and K17 was specific, as signal was not observed using species-specific isotype control antibodies (shown in Figure 3B). Thus, in human ectodermal tissue, AIRE localizes to hair follicle keratinocytes in a pattern partially overlapping with the distribution of K17.
Discussion
In this work, an association between AIRE and K17 was discovered through tandem immunoprecipitation/mass spectrometry and confirmed through co-immunoprecipitation and in situ colocalization. This association was unexpected, as AIRE has classically been characterized as a nuclear protein acting as a transcriptional modulator.5 Investigation of AIRE expression patterns in the HaCaT human epidermal keratinocyte cell line (Figure 2A) and the root sheath of human scalp hair follicles (Figures 2B and 3A) demonstrated filamentous arrangement of AIRE in the cytoplasm with some K17 colocalization. Finally, AIRE was seen to have moderate expression in the developing follicular pegs of human fetal scalp, as well as expression in the nonfollicular epidermis, indicating that AIRE is expressed during hair follicle development. Together, these data indicate a novel expression pattern of AIRE in human epidermal and follicular keratinocytes and suggests an important cytoplasmic role for AIRE independent of its transcriptional activities.
The cytoplasmic localization of AIRE is supported by prior studies on the expression of AIRE-GFP and immunofluorescence detection of wild-type exogenous AIRE in COS-1 cells, where “filamentous” and “tubular” cytoplasmic arrangements have been defined in colocalization with the intermediate filament vimentin.6–8,18 Our data demonstrate that AIRE exists under physiological conditions outside the nucleus in human skin cells and suggest that AIRE associates with the intermediate filament network in epidermal keratinocytes and human hair follicles, perhaps through interactions with K17.
K17 is important for the wound healing response, as well as epithelial cell development and differentiation.14,19 The mutation of K17 in pachyonychia congenita type II and clinical findings of ED in APECED syndrome suggest that the interaction of AIRE and K17 described in the present work may be of pathophysiologic relevance. The CARD domain of AIRE, which is a mutational hotspot for APECED syndrome, has been shown to be functionally important for its cytoplasmic and cytoskeletal localization in COS-1 cells.8 Although the functional significance of AIRE's novel expression pattern and moderate association with K17 in human epidermal and follicular keratinocytes is currently unclear, future studies investigating genotype–phenotype correlations between AIRE CARD mutations and associated ectodermal abnormalities may offer novel insights into the pathogenesis of ectodermal defects in APECED syndrome.
Footnotes
Supported by the Children's Hospital of Philadelphia and Jeffrey Modell Diagnostic Center (J.S.O.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brazil) (L.A.P.), Fundação de Amparo à Pesquisa do Estado de São Paulo grant 09/51747-3 (L.A.P. and A.C.N.), Conselho Nacional de Desenvolvimento Científico e Tecnológico grant 501332/2010-3 (A.C.N.), and the Penn Skin Disease Research Center (NIAMS P30 AR057217).
V.K., L.A.P., A.S.P., and J.S.O. contributed equally to this work.
Contributor Information
Aimee S. Payne, Email: paynea@mail.med.upenn.edu.
Jordan S. Orange, Email: orange@mail.med.upenn.edu.
References
- 1.Vogel A., Strassburg C.P., Obermayer-Straub P., Brabant G., Manns M.P. The genetic background of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy and its autoimmune disease components. J Mol Med. 2002;80:201–211. doi: 10.1007/s00109-001-0306-2. [DOI] [PubMed] [Google Scholar]
- 2.Ahonen P., Myllarniemi S., Sipila I., Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 3.Nagamine K., Peterson P., Scott H.S., Kudoh J., Minoshima S., Heino M., Krohn K.J., Lalioti M.D., Mullis P.E., Antonarakis S.E., Kawasaki K., Asakawa S., Ito F., Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson B.J., Alexander C., Rossi S.W., Liiv I., Rebane A., Worth C.L., Wong J., Laan M., Peterson P., Jenkinson E.J., Anderson G., Scott H.S., Cooke A., Rich T. AIRE's CARD revealed, a new structure for central tolerance provokes transcriptional plasticity. J Biol Chem. 2008;283:1723–1731. doi: 10.1074/jbc.M707211200. [DOI] [PubMed] [Google Scholar]
- 5.Mathis D., Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 6.Pitkanen J., Vahamurto P., Krohn K., Peterson P. Subcellular localization of the autoimmune regulator protein. characterization of nuclear targeting and transcriptional activation domain. J Biol Chem. 2001;276:19597–19602. doi: 10.1074/jbc.M008322200. [DOI] [PubMed] [Google Scholar]
- 7.Rinderle C., Christensen H.M., Schweiger S., Lehrach H., Yaspo M.L. AIRE encodes a nuclear protein co-localizing with cytoskeletal filaments: altered sub-cellular distribution of mutants lacking the PHD zinc fingers. Hum Mol Genet. 1999;8:277–290. doi: 10.1093/hmg/8.2.277. [DOI] [PubMed] [Google Scholar]
- 8.Halonen M., Kangas H., Ruppell T., Ilmarinen T., Ollila J., Kolmer M., Vihinen M., Palvimo J., Saarela J., Ulmanen I., Eskelin P. APECED-causing mutations in AIRE reveal the functional domains of the protein. Hum Mutat. 2004;23:245–257. doi: 10.1002/humu.20003. [DOI] [PubMed] [Google Scholar]
- 9.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A: Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines, J Exp Med 207:299–308 [DOI] [PMC free article] [PubMed]
- 10.Pavlic A., Waltimo-Siren J. Clinical and microstructural aberrations of enamel of deciduous and permanent teeth in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Arch Oral Biol. 2009;54:658–665. doi: 10.1016/j.archoralbio.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Collins S.M., Dominguez M., Ilmarinen T., Costigan C., Irvine A.D. Dermatological manifestations of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome. Br J Dermatol. 2006;154:1088–1093. doi: 10.1111/j.1365-2133.2006.07166.x. [DOI] [PubMed] [Google Scholar]
- 12.Kogawa K., Nagafuchi S., Katsuta H., Kudoh J., Tamiya S., Sakai Y., Shimizu N., Harada M. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80:195–198. doi: 10.1016/s0165-2478(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 13.Sanborn K.B., Rak G.D., Maru S.Y., Demers K., Difeo A., Martignetti J.A., Betts M.R., Favier R., Banerjee P.P., Orange J.S. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol. 2009;182:6969–6984. doi: 10.4049/jimmunol.0804337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan K., Coulombe P.A. The wound repair-associated keratins 6, 16, and 17: Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem. 1998;31:173–204. [PubMed] [Google Scholar]
- 15.Liao H., Sayers J.M., Wilson N.J., Irvine A.D., Mellerio J.E., Baselga E., Bayliss S.J., Uliana V., Fimiani M., Lane E.B., McLean W.H., Leachman S.A., Smith F.J. A spectrum of mutations in keratins K6a: K16 and K17 causing pachyonychia congenita. J Dermatol Sci. 2007;48:199–205. doi: 10.1016/j.jdermsci.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Bonnekoh B., Huerkamp C., Wevers A., Geisel J., Sebok B., Bange F.C., Greenhalgh D.A., Bottger E.C., Krieg T., Mahrle G. Up-regulation of keratin 17 expression in human HaCaT keratinocytes by interferon-gamma. J Invest Dermatol. 1995;104:58–61. doi: 10.1111/1523-1747.ep12613492. [DOI] [PubMed] [Google Scholar]
- 17.McGowan K.M., Coulombe P.A. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J Invest Dermatol. 2000;114:1101–1107. doi: 10.1046/j.1523-1747.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 18.Heino M., Scott H.S., Chen Q., Peterson P., Maebpaa U., Papasavvas M.P., Mittaz L., Barras C., Rossier C., Chrousos G.P., Stratakis C.A., Nagamine K., Kudoh J., Shimizu N., Maclaren N., Antonarakis S.E., Krohn K. Mutation analyses of North American APS-1 patients. Hum Mutat. 1999;13:69–74. doi: 10.1002/(SICI)1098-1004(1999)13:1<69::AID-HUMU8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.McGowan K.M., Coulombe P.A. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998;143:469–486. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee P.P., Pandey R., Zheng R., Suhoski M.M., Monaco-Shawver L., Orange J.S. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]


