Fig. 6.
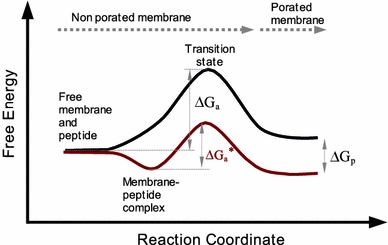
Free energy along the reaction coordinate for opening of a lipidic pore coupled to formation and reorganization of a peptide–membrane complex. Starting from peptide and membrane free species an initial membrane–peptide complex is formed favorably, with peptides adsorbed at the interface. As peptides accumulate, the membrane is stretched asymmetrically, causing thinning and increase of fluctuations. Peptides are postulated to bind more strongly in zones of bilayer defects, corresponding to the transition state, which reduces the activation free energy (∆G a) for lipid reorientation and lipidic pore formation. The ∆G *a energy barrier is expected to decrease with increasing the fraction of membrane-bound peptide up to some threshold value. Additionally, after the pore is formed, binding of the peptide near the pore rim stabilizes it via reduction of the line tension. Notice that the first part of the transformation resembles a catalyzed reaction
