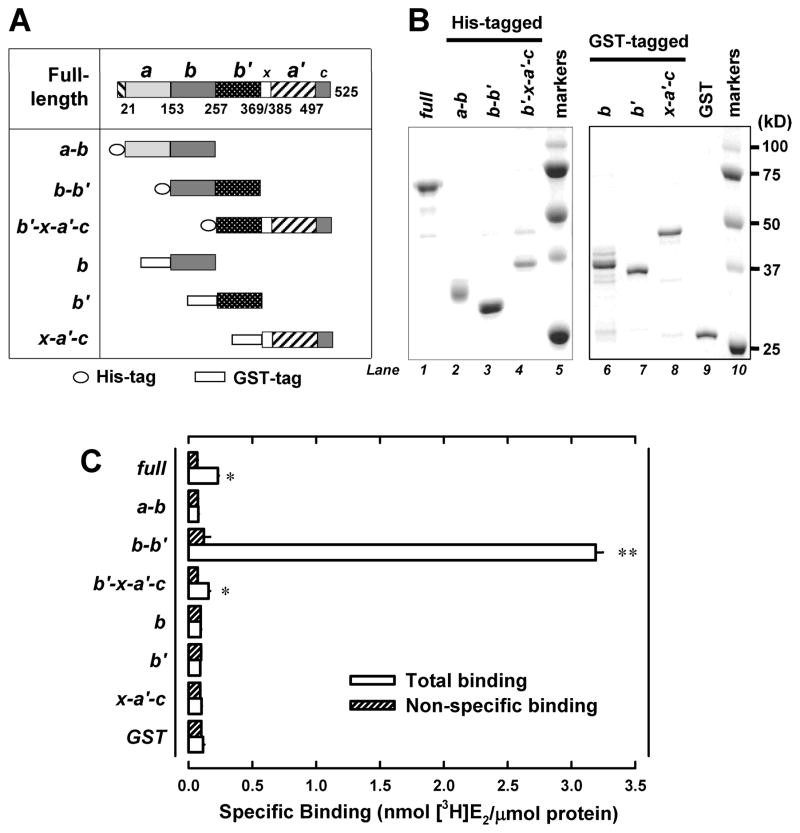
Figure 1. Human PDIp b-b′ fragment contains the E2-binding site.
A. Domain organization of the human PDIp protein (based on Q13087 in the UniProtKB database). Domain boundaries of human PDIp are determined by sequence alignment of PDIp with PDI, whose domain boundaries are described earlier (28). Fragments a-b, b-b′ and b′-x- a′-c were constructed as histidine-tagged fusion proteins. Fragment b, b′ and x-a′-c were constructed as GST-tagged fusion proteins. B. SDS-PAGE analysis of three histidine-tagged PDIp fragments (left part) and three GST-tagged fragments (right part), which were selectively expressed in E. coli cells and then purified using chromatography. C. The binding of [3H]E2 by each of the purified PDIp fragments (at a final concentration of 0.5 μM) after incubation with 4.5 nM [3H]E2 in 10 mM sodium phosphate buffer (pH 7.4) in the absence or presence of 10 μM cold E2. Each value is the mean ± S.D. of triplicate determinations. * P < 0.05, ** P < 0.01, compared to the corresponding non-specific binding. In other experiments, P values were also < 0.05 (for the b′-x-a′-c domain) or < 0.01 (for the b-b′ domain), respectively.