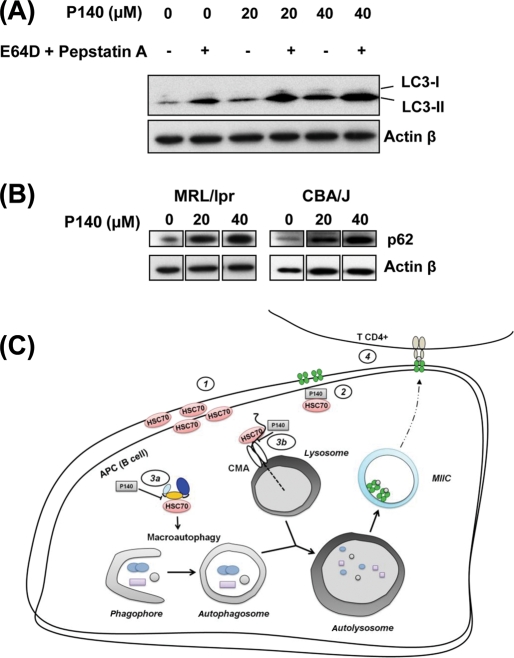
Figure 5.
P140 peptide alters the autophagic pathway by slowing down the lysosomal activity in MRL/lpr B cells. (A) Western immunoblotting analysis with anti-LC3-II antibodies reveals increased appearance of lipidated LC3-II in B cells after 24 h of in vitro P140 treatment. Representative results of two independent experiments. (B) Accumulation of autophagy-selective substrate p62 visualised by Western immunoblotting in MRL/lpr or CBA/J B cells treated in vitro with P140. Representative results of two independent experiments. (C) A model by which P140 peptide interacts with HSC70, alters its properties and influences activation of autoreactive lymphocytes in lupus. (1) HSC70 is overexpressed both at the surface and intracellularly on lupus antigen-presenting cells (APC; B cells). (2) P140 hampers/inhibits chaperone properties of HSC70 protein. P140 (3a) disturbs a complex of co-chaperones involved in the regulation of macroautophagy (putative) and slows down the autophagic flux, and/or (3b) affects chaperone-mediated autophagy (CMA) leading to the alteration of (auto)antigens processing by lysosomal hydrolysis. (4) Subsequent delivery of antigens into MIIC compartments for class II presentation is altered and class II MHC molecules are destabilised, therefore compromising autoreactive CD4 T-cell response in a beneficial pathway for the P40-treated individual.